Ground State Electron Configuration For Bromine
listenit
Mar 26, 2025 · 6 min read
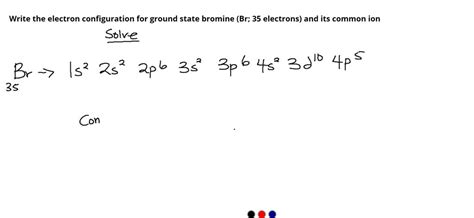
Table of Contents
Ground State Electron Configuration for Bromine: A Deep Dive
Bromine, a fascinating element residing in Group 17 of the periodic table, presents a compelling case study for understanding electron configuration. Its position, as a halogen, dictates its chemical behavior and significantly influences its electronic structure. This article delves into the ground state electron configuration of bromine, exploring its derivation, implications, and relevance to its properties. We'll also touch upon the nuances of electron configuration and its crucial role in chemistry.
Understanding Electron Configuration
Before diving into bromine's specifics, let's establish a foundational understanding of electron configuration itself. Electron configuration describes the arrangement of electrons in the various energy levels (shells) and sublevels (subshells) within an atom. It's a fundamental concept in chemistry, as it dictates an atom's chemical reactivity, bonding behavior, and overall properties.
Principles Governing Electron Configuration
Several principles guide the filling of electrons into orbitals:
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level and progressing upwards. This principle dictates the order in which subshells are filled.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, with opposite spins. This means each orbital can contain at most one electron with spin up and one electron with spin down.
- Hund's Rule: When multiple orbitals of the same energy (degenerate orbitals) are available, electrons will individually occupy each orbital before pairing up in any one orbital. This maximizes the total spin and enhances stability.
Notation and Representation
Electron configurations are typically represented using a concise notation that specifies the principal quantum number (n), the subshell (s, p, d, or f), and the number of electrons in each subshell. For example, 1s² indicates two electrons in the 1s subshell. The superscript represents the number of electrons.
Deriving the Ground State Electron Configuration of Bromine (Br)
Bromine has an atomic number of 35, meaning it possesses 35 protons and, in its neutral state, 35 electrons. Following the Aufbau principle, Hund's rule, and the Pauli exclusion principle, we can systematically fill the orbitals:
- 1s²: The first two electrons fill the lowest energy level, the 1s subshell.
- 2s²: The next two electrons fill the 2s subshell.
- 2p⁶: The next six electrons fill the three 2p orbitals (2px, 2py, 2pz), each with two electrons (following Hund's rule).
- 3s²: Two electrons fill the 3s subshell.
- 3p⁶: Six electrons fill the three 3p orbitals.
- 4s²: Two electrons fill the 4s subshell. Note that the 4s subshell fills before the 3d subshell due to its lower energy.
- 3d¹⁰: Ten electrons fill the five 3d orbitals.
- 4p⁵: Five electrons fill the three 4p orbitals, one electron short of a filled subshell.
Therefore, the complete ground state electron configuration for bromine is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵.
Noble Gas Configuration
To simplify the notation, we can use the noble gas configuration. Bromine follows Argon (Ar) in the periodic table. Argon has an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶. We can write bromine's configuration as: [Ar] 4s² 3d¹⁰ 4p⁵. This shortened notation clearly shows that bromine has a filled Argon core and additional electrons in the 4s, 3d, and 4p subshells.
Implications of Bromine's Electron Configuration
Bromine's electron configuration has profound implications for its chemical properties and behavior:
-
Reactivity: The presence of seven electrons in the 4p subshell means bromine is one electron short of achieving a stable noble gas configuration (like krypton). This makes it highly reactive, readily gaining an electron to form a bromide ion (Br⁻) with a stable octet. This drive to achieve a full octet underpins many of bromine's chemical reactions.
-
Bonding: Bromine readily forms covalent bonds by sharing electrons with other atoms, achieving a stable octet. It often forms single bonds and exhibits a valence of -1, although higher oxidation states are possible in certain compounds.
-
Oxidation States: While bromine commonly exhibits a -1 oxidation state (in bromides), it can also exhibit positive oxidation states (+1, +3, +5, +7) in compounds with more electronegative elements like oxygen and fluorine. This is because in these compounds, bromine can share or lose electrons from its 4s and 4p orbitals, showcasing its versatility in bonding.
-
Physical Properties: The electronic structure influences several physical properties, such as its boiling point and melting point. The relatively strong intermolecular forces (van der Waals forces) arising from the electron distribution influence these properties.
Bromine's Role in Chemistry and Beyond
Bromine plays a significant role in various chemical applications and industrial processes:
-
Inorganic Chemistry: Bromine is used in the production of various inorganic bromides, which find use in diverse applications, including flame retardants, water purification, and photography.
-
Organic Chemistry: Bromine is used as a reagent in organic synthesis for bromination reactions, introducing bromine atoms into organic molecules. This has applications in the pharmaceutical, agricultural, and materials science industries.
-
Industrial Applications: It's utilized in the production of certain dyes, pesticides, and other chemical compounds. Bromine compounds are also increasingly used in various industrial applications.
-
Environmental Considerations: The use of bromine and its compounds raises some environmental concerns, particularly regarding their potential impact on the ozone layer (certain brominated compounds have been identified as ozone-depleting substances) and aquatic life. Therefore, responsible use and disposal of bromine-containing materials are crucial.
Further Exploration: Excited State Electron Configurations
The ground state configuration represents the lowest energy arrangement of electrons. However, when an atom absorbs energy, an electron can jump to a higher energy level, resulting in an excited state electron configuration. These excited states are transient and typically revert to the ground state, emitting energy in the process. The excited state electron configuration of bromine would involve promoting an electron to a higher energy subshell. This transition significantly impacts the atom's chemical reactivity and potential bonding behavior.
Conclusion: The Significance of Electron Configuration
The ground state electron configuration of bromine (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵ or [Ar] 4s² 3d¹⁰ 4p⁵) is central to understanding its chemical properties and behavior. The presence of seven electrons in the outermost shell drives its reactivity and its tendency to form either ionic or covalent bonds. This seemingly simple configuration underlies the element's diverse applications and the complexities of its interactions with other elements. By understanding electron configuration, we unlock the key to predicting chemical behavior and interpreting the macroscopic properties of matter. Further research into bromine's various compounds and its role in different chemical processes would reveal a deeper appreciation for the impact of its electron configuration.
Latest Posts
Latest Posts
-
What Is 2 5 As A Decimal
Mar 29, 2025
-
18 As A Percentage Of 60
Mar 29, 2025
-
Is Hydrogen Gas At Room Temperature
Mar 29, 2025
-
Solving A System By The Addition Method
Mar 29, 2025
-
What Is Half Of A Half Teaspoon
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Ground State Electron Configuration For Bromine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
