What Is The Charge Of An Oxygen Ion
listenit
Mar 18, 2025 · 5 min read
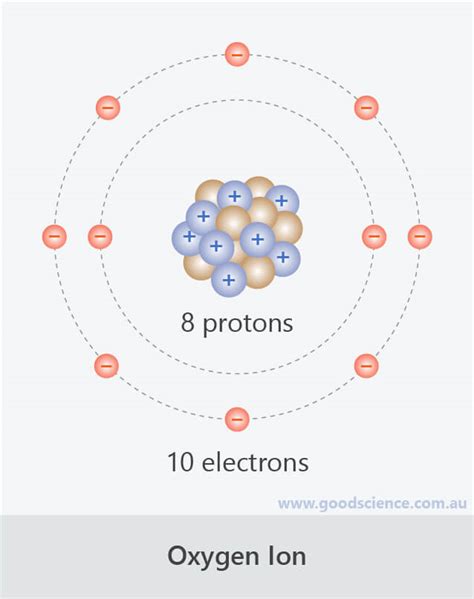
Table of Contents
What is the Charge of an Oxygen Ion? A Deep Dive into Ionic Bonding and Oxidation States
Oxygen, a cornerstone element of life and a highly reactive nonmetal, doesn't exist independently as a neutral atom in many common compounds. Instead, it readily gains electrons to achieve a stable electron configuration, forming negatively charged ions called oxide anions. Understanding the charge of an oxygen ion is fundamental to comprehending chemical bonding, reactivity, and the properties of countless materials. This article will delve deep into the intricacies of oxygen's ionic charge, exploring its electron configuration, its role in ionic bonding, and the exceptions to the general rule.
Oxygen's Electron Configuration and the Octet Rule
To understand why oxygen forms a negatively charged ion, we must examine its electron configuration. Oxygen (O) has an atomic number of 8, meaning it possesses 8 protons and 8 electrons in its neutral state. Its electronic structure is 1s²2s²2p⁴. The outermost electron shell (the valence shell) contains six electrons (two in the 2s subshell and four in the 2p subshell).
The octet rule, a crucial principle in chemistry, states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their valence shell, mimicking the stable electron configuration of noble gases. For oxygen, achieving this stable octet requires gaining two electrons. This gain of two negatively charged electrons results in a net negative charge of 2-.
The Formation of the Oxide Anion (O²⁻)
The process of oxygen gaining two electrons is a reduction reaction. This means oxygen accepts electrons, decreasing its overall oxidation state. When oxygen gains these two electrons, it transforms into the oxide anion, denoted as O²⁻. This anion is highly stable due to its complete octet.
Example: Consider the formation of magnesium oxide (MgO). Magnesium (Mg) has two valence electrons and readily loses them to achieve a stable octet, forming a 2+ cation (Mg²⁺). Oxygen, needing two electrons to complete its octet, readily accepts these two electrons from magnesium. The electrostatic attraction between the positively charged magnesium cation and the negatively charged oxide anion forms the ionic compound magnesium oxide.
Mg + O → Mg²⁺ + O²⁻ → MgO
Oxidation States and the Charge of Oxygen
While the charge of the oxide anion is consistently 2-, the concept of oxidation state adds a layer of complexity. Oxidation state is a number assigned to an atom representing the number of electrons lost or gained by that atom in a compound. It's a bookkeeping system to track electron transfers in chemical reactions.
While the oxide ion (O²⁻) always has a -2 charge, the oxidation state of oxygen can deviate from -2 in certain exceptional cases. This includes:
-
Peroxides: In peroxides (e.g., hydrogen peroxide, H₂O₂), each oxygen atom has an oxidation state of -1. This is because the oxygen atoms are bonded to each other, sharing electrons differently than in typical oxides. The oxygen-oxygen bond involves a single bond, leaving each oxygen atom with only one electron gained instead of two.
-
Superoxides: Superoxides (e.g., potassium superoxide, KO₂) contain oxygen in a -½ oxidation state. This arises from the unique oxygen-oxygen bonding in these compounds.
-
Fluorides: In oxygen fluorides (e.g., OF₂, oxygen difluoride), fluorine, being the most electronegative element, pulls electron density away from oxygen. In this case, oxygen exhibits a positive oxidation state (+2 in OF₂). This is unusual for oxygen and highlights fluorine's exceptional electronegativity.
-
Ozone (O₃): In ozone, a molecule of elemental oxygen, the oxidation state of each oxygen atom is zero. This is because ozone is a molecule composed of only oxygen atoms, with no net electron transfer.
The Importance of Understanding Oxygen's Charge
Understanding the charge of the oxygen ion and its various oxidation states is crucial in many areas of chemistry and related fields. Here are some key applications:
-
Predicting the Properties of Ionic Compounds: The charge of the oxygen ion influences the physical and chemical properties of ionic compounds, including their melting points, boiling points, solubility, and reactivity. The strong electrostatic forces between the positively and negatively charged ions in ionic compounds lead to high melting and boiling points, for example.
-
Balancing Chemical Equations: Knowing the charge of oxygen is essential for correctly balancing redox reactions, where electron transfer occurs.
-
Understanding Redox Reactions: Redox reactions involve a change in oxidation state, and the role of oxygen in these reactions is often key. Oxygen's tendency to gain electrons and form oxide anions drives many oxidation processes.
-
Material Science: Oxygen's role in forming oxides plays a vital role in material science. Understanding the ionic interactions in these materials allows for tailoring their properties for specific applications.
-
Environmental Chemistry: Oxygen's involvement in processes like combustion, oxidation, and corrosion is crucial in understanding environmental chemistry, air pollution, and water treatment.
-
Biological Systems: Oxygen is essential for respiration, the process by which living organisms convert energy from food. The chemistry of oxygen's interactions within biological systems hinges on its ability to gain electrons and become reduced.
Beyond the Basics: More Complex Scenarios
While the -2 charge is dominant for oxygen, the exceptions mentioned above demonstrate the nuanced nature of chemical bonding. Factors like electronegativity, the presence of other atoms, and bonding type significantly influence the effective charge and oxidation state of oxygen. Advanced concepts like formal charge and resonance structures are often necessary to fully describe the electron distribution in molecules containing oxygen.
Conclusion
The charge of an oxygen ion, primarily -2 as an oxide anion (O²⁻), is a fundamental concept in chemistry. This negative charge arises from oxygen's tendency to gain two electrons to complete its octet, driven by its high electronegativity. While the -2 charge is most common, exceptions exist in peroxides, superoxides, oxygen fluorides, and other unusual compounds. Understanding oxygen's charge, alongside its oxidation states, is paramount for comprehending chemical bonding, reactivity, and the properties of numerous compounds and materials. This knowledge is invaluable across numerous disciplines, underscoring the fundamental importance of this seemingly simple aspect of oxygen's chemistry. Further exploration into advanced bonding theories and computational chemistry provides a more complete picture of electron distribution and charge in complex oxygen-containing molecules and materials.
Latest Posts
Latest Posts
-
Graph 2 X 1 2 3
Mar 18, 2025
-
How Many Ius In A Milligram
Mar 18, 2025
-
What Group Is The Most Reactive Metals
Mar 18, 2025
-
How Many Square Inches In 1 Square Foot
Mar 18, 2025
-
What Does The Atomic Number Tell You About The Element
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of An Oxygen Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
