What Group Is The Most Reactive Metals
listenit
Mar 18, 2025 · 5 min read
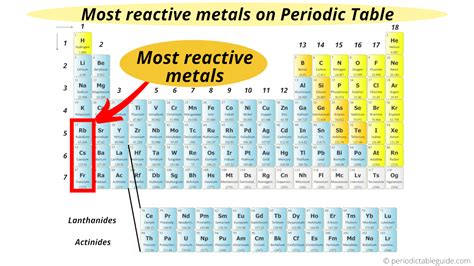
Table of Contents
What Group is the Most Reactive Metals? Understanding Alkali Metals and Reactivity
The periodic table organizes elements based on their properties, and one of the most striking trends is the variation in reactivity. Among metals, the alkali metals (Group 1) are undisputed champions of reactivity. This article will delve deep into understanding why alkali metals are the most reactive, exploring their electronic configurations, chemical reactions, and practical applications, while also touching upon the factors that influence reactivity within the group itself.
Understanding Reactivity: A Look at Electron Configuration
Chemical reactivity, at its core, is determined by an element's tendency to gain, lose, or share electrons to achieve a stable electron configuration. Atoms strive for the stability of a noble gas configuration, possessing a full outermost electron shell (valence shell). This is often referred to as the octet rule, though exceptions exist, particularly for elements beyond the third period.
Alkali metals, located in Group 1 of the periodic table, possess a single electron in their outermost shell. This lone valence electron is relatively loosely held, making it easy to lose. Losing this electron results in a stable, positively charged ion with a full outermost shell, mimicking the electronic structure of the noble gas in the preceding period. This ease of electron loss is the fundamental reason behind the high reactivity of alkali metals.
Comparing Alkali Metals to Other Groups
Consider other groups of metals:
-
Alkaline Earth Metals (Group 2): These metals have two valence electrons. While they are also reactive, they require more energy to lose two electrons compared to the alkali metals losing one. This makes them significantly less reactive than alkali metals.
-
Transition Metals: Transition metals exhibit variable oxidation states, meaning they can lose varying numbers of electrons. Their reactivity is generally lower than alkali metals because the valence electrons are more tightly bound to the nucleus. Furthermore, the presence of d-electrons adds complexity to their reactivity patterns.
-
Post-Transition Metals: These metals show a wider range of reactivity, but generally less than alkali metals. Their electronic configurations are more complex, and their reactivity is influenced by several factors, including shielding effects and electronegativity.
The Chemical Reactions of Alkali Metals: A Testament to Reactivity
The reactivity of alkali metals manifests in several dramatic and characteristic ways:
Reaction with Water: A Violent Affair
Alkali metals react vigorously with water, producing hydrogen gas and a metal hydroxide. The reaction becomes increasingly violent as you go down the group. Lithium reacts moderately, producing a steady stream of bubbles. Sodium reacts quite rapidly, generating significant heat and often igniting the produced hydrogen gas. Potassium's reaction is even more vigorous, resulting in a bright flame. Rubidium and cesium react explosively, even with moisture in the air.
The equation for the general reaction is:
2M(s) + 2H₂O(l) → 2MOH(aq) + H₂(g)
Where M represents the alkali metal.
Reaction with Halogens: Salt Formation
Alkali metals readily react with halogens (Group 17 elements) to form ionic compounds known as halides. These reactions are extremely exothermic (release a significant amount of heat). For example, the reaction of sodium with chlorine produces sodium chloride (common table salt):
2Na(s) + Cl₂(g) → 2NaCl(s)
This reaction illustrates the strong tendency of alkali metals to lose an electron and halogens to gain one, resulting in a stable ionic compound with strong electrostatic forces holding the ions together.
Reaction with Oxygen: Oxide Formation
The reaction with oxygen varies depending on the alkali metal. Lithium forms lithium oxide (Li₂O), while sodium can form sodium peroxide (Na₂O₂) and sodium superoxide (NaO₂). Potassium, rubidium, and cesium primarily form superoxides. The differences arise from the increasing size of the metal atoms as you descend the group and the influence of the size and electronegativity of oxygen.
Factors Affecting Reactivity within Group 1
While all alkali metals are highly reactive, there are subtle differences in their reactivity. This variation is primarily attributed to two factors:
Atomic Radius: The Distance Matters
As you move down Group 1, the atomic radius increases. This means the outermost electron is further away from the positively charged nucleus and experiences less electrostatic attraction. This weaker attraction makes it easier to lose the electron, resulting in an increase in reactivity. Hence, cesium, with the largest atomic radius, is the most reactive alkali metal.
Ionization Energy: The Energy of Removal
Ionization energy is the energy required to remove an electron from an atom. As the atomic radius increases down the group, the ionization energy decreases. Lower ionization energy implies it takes less energy to remove the valence electron, confirming the trend of increased reactivity as you move down the group.
Practical Applications Leveraging Alkali Metal Reactivity
The remarkable reactivity of alkali metals makes them valuable in various applications:
-
Lithium-ion batteries: Lithium's high reactivity and low atomic weight make it ideal for use in rechargeable batteries found in many electronic devices and electric vehicles.
-
Sodium lamps: Sodium's intense yellow light emission is used in streetlights and other lighting applications.
-
Potassium in fertilizers: Potassium compounds are essential nutrients for plant growth, and are crucial components of many fertilizers.
-
Cesium in atomic clocks: Cesium's precise atomic transitions are used to create highly accurate atomic clocks.
Safety Precautions When Handling Alkali Metals
Because of their high reactivity, alkali metals pose significant safety hazards. They should be handled with extreme caution and appropriate safety measures:
-
Never expose alkali metals to water or moisture: This can lead to violent reactions and potentially dangerous explosions.
-
Store alkali metals under inert atmospheres: Inert gases like argon prevent them from reacting with oxygen or moisture in the air.
-
Always wear appropriate personal protective equipment (PPE): This includes gloves, eye protection, and possibly a lab coat.
Conclusion: The Reign of the Alkali Metals
Alkali metals stand out as the most reactive group of metals due to their electronic configuration, featuring a single loosely held valence electron. This readily lost electron is responsible for their vigorous reactions with water, halogens, and oxygen. While all alkali metals are highly reactive, the reactivity increases as you move down the group due to increasing atomic radius and decreasing ionization energy. Understanding the unique reactivity of alkali metals is critical for their safe handling and their effective application in various technologies and industries. Their inherent reactivity, while posing safety challenges, also underpins their significant contributions to modern technology and everyday life.
Latest Posts
Latest Posts
-
What Is The Greatest Common Factor Of 60 And 36
Mar 18, 2025
-
Distance From Nashville To Atlanta Georgia
Mar 18, 2025
-
Calculate The Ph At The Equivalence Point
Mar 18, 2025
-
64 Ounces Equals How Many Pounds
Mar 18, 2025
-
How To Find Valence Electrons Of Transition Elements
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Group Is The Most Reactive Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
