What Is Polymer Of Amino Acids
listenit
Mar 18, 2025 · 6 min read
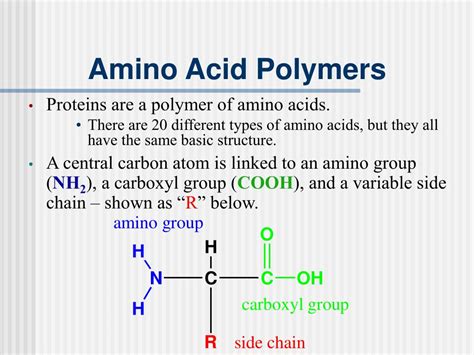
Table of Contents
What is a Polymer of Amino Acids? Understanding Proteins and Their Significance
Proteins, the workhorses of life, are fundamental to virtually every biological process. From catalyzing reactions to providing structural support, their diverse roles are crucial for the survival and function of all living organisms. But what exactly are proteins? At their core, proteins are polymers of amino acids, meaning they are long chains of smaller molecules called amino acids linked together in a specific sequence. Understanding this fundamental concept opens the door to grasping the complexity and importance of proteins in biology.
The Building Blocks: Amino Acids
Before delving into the intricacies of protein structure, let's first examine the individual components: amino acids. These are organic molecules containing a central carbon atom (the alpha-carbon) bonded to four different chemical groups:
- An amino group (-NH2): This group is basic and readily accepts a proton (H+).
- A carboxyl group (-COOH): This group is acidic and readily donates a proton (H+).
- A hydrogen atom (-H): A simple hydrogen atom.
- A variable side chain (R group): This is the unique part of each amino acid, determining its properties and how it interacts with other amino acids.
There are 20 standard amino acids that are commonly found in proteins, each with a different R group. These R groups can be hydrophobic (water-repelling), hydrophilic (water-attracting), charged (positive or negative), or even contain special functional groups like hydroxyl (-OH) or sulfhydryl (-SH) groups. This diversity in R groups is what gives proteins their incredible structural and functional diversity.
Properties of Amino Acids Influencing Protein Structure
The properties of the R groups profoundly impact the final three-dimensional structure of a protein. For example:
- Hydrophobic amino acids tend to cluster together in the protein's interior, away from the surrounding water molecules.
- Hydrophilic amino acids often reside on the protein's surface, interacting with the aqueous environment.
- Charged amino acids can form ionic bonds with each other, stabilizing protein structure.
The Peptide Bond: Linking Amino Acids
Amino acids are linked together through a process called dehydration synthesis or condensation reaction. In this reaction, the carboxyl group of one amino acid reacts with the amino group of another amino acid, releasing a water molecule (H2O) and forming a peptide bond. This peptide bond is a covalent bond, a strong linkage that holds the amino acids together in a chain.
The resulting chain of amino acids is called a polypeptide. A protein can consist of one or more polypeptide chains, each with a unique sequence of amino acids. The sequence of amino acids in a polypeptide is determined by the genetic code, encoded in DNA. This sequence dictates the protein's three-dimensional structure and, consequently, its function.
Levels of Protein Structure: From Linear Chain to Functional Form
The complexity of a protein's structure extends beyond the simple linear sequence of amino acids. Proteins exhibit four hierarchical levels of structure:
1. Primary Structure: The Amino Acid Sequence
The primary structure is simply the linear sequence of amino acids in a polypeptide chain. This sequence is dictated by the genetic code and is crucial because it determines all higher levels of protein structure. Even a single amino acid substitution can drastically alter the protein's function, as famously illustrated by sickle cell anemia, caused by a single amino acid change in hemoglobin.
2. Secondary Structure: Local Folding Patterns
The secondary structure refers to local folding patterns within the polypeptide chain. These patterns are stabilized by hydrogen bonds between the amino and carboxyl groups of the peptide backbone. Two common secondary structures are:
- Alpha-helices: A right-handed coiled structure stabilized by hydrogen bonds between every fourth amino acid.
- Beta-sheets: Extended, pleated structures formed by hydrogen bonds between adjacent polypeptide chains or segments of the same chain.
Other secondary structures, like loops and turns, also contribute to the overall protein architecture.
3. Tertiary Structure: The 3D Arrangement
The tertiary structure describes the overall three-dimensional arrangement of a single polypeptide chain. This structure is stabilized by a variety of interactions, including:
- Disulfide bonds: Covalent bonds between cysteine residues.
- Hydrogen bonds: Weaker bonds between various polar groups.
- Hydrophobic interactions: Interactions between nonpolar side chains.
- Ionic bonds (salt bridges): Electrostatic interactions between charged side chains.
The tertiary structure is crucial for the protein's function, as it determines the arrangement of its active sites (in enzymes), binding sites (in receptors), or other functional regions.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins are composed of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these individual subunits is called the quaternary structure. These subunits can be identical or different, and their interactions are essential for the protein's overall function. Hemoglobin, for example, is a tetramer composed of four subunits.
The Importance of Protein Structure and Function
The precise three-dimensional structure of a protein is intimately linked to its function. Any disruption or alteration of this structure, often caused by changes in temperature, pH, or the presence of certain chemicals (denaturation), can lead to loss of function.
The diverse functions of proteins arise from their unique structures and the specific interactions they engage in:
- Enzymes: Catalyze biochemical reactions, speeding up metabolic processes.
- Structural proteins: Provide support and shape to cells and tissues (e.g., collagen, keratin).
- Transport proteins: Carry molecules across cell membranes or throughout the body (e.g., hemoglobin).
- Motor proteins: Generate movement within cells or the organism (e.g., myosin, kinesin).
- Hormones: Act as chemical messengers, regulating various physiological processes (e.g., insulin, glucagon).
- Antibodies: Part of the immune system, defending against pathogens.
- Receptor proteins: Bind to specific molecules and trigger cellular responses.
Studying Proteins: Techniques and Applications
The study of proteins, known as proteomics, is a vast and rapidly evolving field. Numerous techniques are employed to understand protein structure, function, and interactions:
- X-ray crystallography: Determines the three-dimensional structure of proteins by analyzing diffraction patterns of X-rays passed through protein crystals.
- Nuclear magnetic resonance (NMR) spectroscopy: Provides information about protein structure and dynamics in solution.
- Mass spectrometry: Identifies and quantifies proteins in a sample.
- Chromatography: Separates proteins based on their properties, such as size, charge, or hydrophobicity.
- Gel electrophoresis: Separates proteins based on their size and charge.
These techniques are crucial for advancements in various fields:
- Drug discovery and development: Understanding protein structure and function is essential for designing drugs that target specific proteins involved in disease.
- Diagnostics: Protein analysis is used to diagnose various diseases, such as cancer and infectious diseases.
- Biotechnology: Proteins are used in numerous biotechnological applications, such as producing biofuels, enzymes for industrial processes, and therapeutic proteins.
- Food science: Protein analysis is used to improve the quality and nutritional value of food products.
Conclusion: The Significance of Amino Acid Polymers
Proteins, the polymers of amino acids, are the fundamental building blocks and functional units of life. Their remarkable diversity in structure and function stems from the 20 standard amino acids and the vast number of possible sequences and folding patterns they can adopt. Understanding the intricate relationship between amino acid sequence, protein structure, and function is crucial for advancements in biology, medicine, and biotechnology. The ongoing research in proteomics continues to unravel the complexities of these remarkable molecules, revealing new insights into the mechanisms of life and paving the way for innovative applications in diverse fields.
Latest Posts
Latest Posts
-
Why Is The Replication Of Dna Called Semiconservative
Mar 18, 2025
-
How Many P Orbitals Are There
Mar 18, 2025
-
How Do You Find The Perpendicular Slope
Mar 18, 2025
-
How To Find The Domain Restrictions
Mar 18, 2025
-
What Is Half Of 1 1 4
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is Polymer Of Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
