How Many P Orbitals Are There
listenit
Mar 18, 2025 · 6 min read
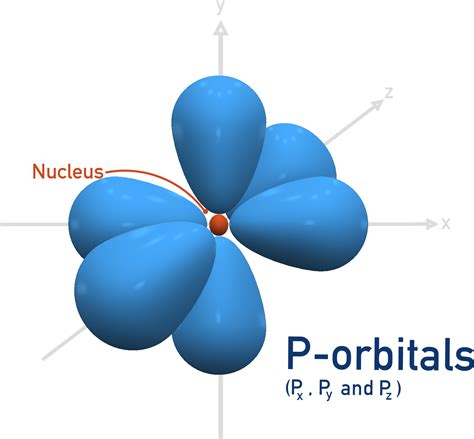
Table of Contents
How Many p Orbitals Are There? A Deep Dive into Atomic Orbitals
Understanding atomic orbitals is fundamental to grasping the behavior of atoms and molecules. This article delves into the specifics of p orbitals, exploring their shape, energy levels, and the crucial role they play in chemical bonding. We’ll answer the core question: how many p orbitals are there? But we’ll go far beyond that simple answer, providing a comprehensive overview of p orbitals and their significance in chemistry.
What are Atomic Orbitals?
Before we dive into p orbitals specifically, let's establish a clear understanding of atomic orbitals in general. An atomic orbital is a region of space around an atom's nucleus where there's a high probability of finding an electron. It's crucial to understand that we cannot pinpoint an electron's exact location; instead, we describe its probable location using these orbitals. These orbitals are defined by quantum numbers, which provide a mathematical description of an electron's state.
The key quantum numbers influencing an orbital's characteristics are:
-
Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It can be any positive integer (1, 2, 3, etc.). Higher values of 'n' correspond to higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number defines the shape of the orbital and its angular momentum. It can have integer values ranging from 0 to n-1. For example, if n=2, l can be 0 or 1.
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can have integer values ranging from -l to +l, including 0.
Understanding the p Orbitals: Shape and Orientation
Now, let's focus on p orbitals. These orbitals are characterized by an azimuthal quantum number (l) of 1. This means that for a given principal quantum number (n), there are three possible p orbitals, differentiated by their magnetic quantum number (ml):
- ml = -1: This corresponds to the px orbital.
- ml = 0: This corresponds to the py orbital.
- ml = +1: This corresponds to the pz orbital.
Each p orbital has a dumbbell shape, with two lobes extending in opposite directions from the nucleus. The three p orbitals are oriented along the x, y, and z axes of a Cartesian coordinate system, hence their designations px, py, and pz. This spatial orientation is crucial for their role in forming chemical bonds.
Visualizing the p Orbitals
Imagine three dumbbells positioned perpendicularly to each other. One lies along the x-axis (px), another along the y-axis (py), and the third along the z-axis (pz). This is a simplified, but helpful, visualization of the three p orbitals. It's important to remember that these are probability distributions; the electron is not confined to the lobes themselves but has a probability of being found within the entire orbital.
Energy Levels of p Orbitals
Within a given principal energy level (n), the three p orbitals (px, py, and pz) have the same energy. They are said to be degenerate. This degeneracy is lifted (broken) when the atom is subjected to an external electric or magnetic field. However, in the absence of such external fields, they possess identical energy levels.
This degeneracy is a key factor in understanding chemical bonding. Because the p orbitals have equal energy, electrons fill them equally before moving to higher energy levels. This follows Hund's rule, which states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital.
The Role of p Orbitals in Chemical Bonding
p orbitals are essential players in chemical bonding, particularly in the formation of covalent bonds. The overlap of p orbitals between atoms leads to the formation of pi (π) bonds, which are crucial for the stability and reactivity of many molecules. For example:
-
Multiple Bonds: Multiple bonds (double and triple bonds) often involve the overlap of p orbitals. A double bond typically consists of one sigma (σ) bond and one pi (π) bond, while a triple bond comprises one sigma (σ) bond and two pi (π) bonds.
-
Organic Chemistry: The chemistry of carbon is largely defined by its four valence electrons, two in the 2s orbital and two in the 2p orbitals. The ability of carbon to form four bonds, creating diverse organic molecules, is directly tied to its p orbitals.
-
Inorganic Chemistry: Many inorganic compounds also exhibit bonding involving p orbitals. Transition metal complexes, for example, frequently involve the participation of d and p orbitals in complex bonding schemes.
How Many p Orbitals Are There – A More Nuanced Answer
So, we've established that for a given principal quantum number (n), there are three p orbitals. But the question, "How many p orbitals are there?" is slightly more nuanced than it initially seems. The answer depends on the energy level we're considering.
Since the principal quantum number (n) can be any positive integer (1, 2, 3,...), theoretically, there are an infinite number of p orbitals. However, in practice, we rarely encounter atoms with electrons occupying p orbitals beyond the seventh principal energy level (n=7) under normal conditions.
Therefore, while the theoretical number is infinite, the practical number is limited by the number of electrons an atom possesses and the energy levels it can populate. For most chemical considerations, focusing on the three p orbitals within a given principal energy level (n) is sufficient for a complete understanding.
Beyond the Basics: Hybrid Orbitals and Molecular Orbital Theory
The discussion above provides a foundational understanding of p orbitals. However, a more complete picture requires considering the concepts of hybrid orbitals and molecular orbital theory.
Hybrid Orbitals
In many molecules, atomic orbitals combine to form hybrid orbitals. This hybridization involves the mixing of s and p orbitals to create new orbitals with different shapes and energies, optimized for bonding. For instance, sp3, sp2, and sp hybridized orbitals are common in organic chemistry, significantly impacting molecular geometry and reactivity.
Molecular Orbital Theory
Molecular orbital theory provides a more sophisticated approach to understanding bonding. Instead of considering atomic orbitals, this theory focuses on molecular orbitals, which are formed by the combination of atomic orbitals from different atoms. The interaction of p atomic orbitals leads to the formation of bonding and antibonding molecular orbitals, influencing the overall stability and electronic properties of the molecule.
p Orbitals and Spectroscopy
The properties of p orbitals are directly observable through various spectroscopic techniques. For instance:
-
UV-Vis Spectroscopy: Electronic transitions involving p orbitals are frequently observed in the ultraviolet-visible region of the electromagnetic spectrum. The absorption or emission of light at specific wavelengths provides information about the energy levels of the p orbitals and the nature of chemical bonds.
-
Photoelectron Spectroscopy (PES): PES provides detailed information on the ionization energies of electrons, including those occupying p orbitals. This allows for precise determination of orbital energies and provides insights into electron distribution within the atom or molecule.
Conclusion: The Enduring Importance of p Orbitals
The seemingly simple question of how many p orbitals exist opens a door to a rich and complex understanding of atomic structure and chemical bonding. While the theoretical number is infinite, the practical relevance primarily lies within the three p orbitals per principal quantum level (n). These orbitals are fundamental to chemical reactivity, molecular geometry, and numerous spectroscopic techniques. Their role is central to the vast world of chemistry, from simple organic molecules to complex inorganic compounds and beyond. Therefore, a deep grasp of p orbitals is crucial for anyone looking to understand the fundamental workings of the atomic world and the molecules it builds.
Latest Posts
Latest Posts
-
How Does Temperature Affect Cellular Respiration
Mar 18, 2025
-
Why Is The Atomic Mass Not A Whole Number
Mar 18, 2025
-
What Type Of Rock Are Fossils Usually Found In
Mar 18, 2025
-
Is The Sun Abiotic Or Biotic
Mar 18, 2025
-
Greatest Common Factor Of 36 And 27
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many P Orbitals Are There . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
