What Is Electronic Configuration Of Carbon
listenit
Mar 21, 2025 · 6 min read
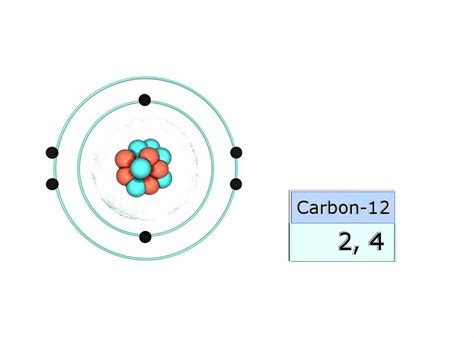
Table of Contents
What is the Electronic Configuration of Carbon? A Deep Dive
Carbon, the cornerstone of organic chemistry and the building block of life, possesses a deceptively simple yet profoundly influential electronic configuration. Understanding this configuration is key to unlocking its remarkable versatility and explaining its diverse chemical behavior. This article will delve deep into the electronic configuration of carbon, exploring its implications for bonding, reactivity, and the vast array of molecules it forms.
The Basics: Atomic Number and Electron Shells
Carbon's atomic number is 6, meaning it possesses six protons in its nucleus and, in its neutral state, six electrons orbiting around it. These electrons are not randomly scattered but occupy specific energy levels or shells, arranged according to the principles of quantum mechanics. The arrangement of these electrons determines the atom's chemical properties and its ability to form bonds with other atoms.
The Shell Model: A Simplified Representation
A simplified way to visualize this is through the shell model. The first shell, closest to the nucleus, can hold a maximum of two electrons. The second shell can accommodate up to eight electrons. For carbon, we have:
- Shell 1 (K shell): 2 electrons
- Shell 2 (L shell): 4 electrons
This arrangement leads to carbon's electronic configuration, which is typically represented as 1s²2s²2p². Let's break down this notation:
- 1s²: This indicates two electrons in the 1s orbital. The '1' represents the principal quantum number (energy level), 's' denotes the subshell type (spherical), and the superscript '2' specifies the number of electrons in that orbital.
- 2s²: Two electrons reside in the 2s orbital. Again, '2' is the principal quantum number, 's' the subshell type, and '2' the number of electrons.
- 2p²: Two electrons occupy the 2p subshell. The '2' indicates the principal quantum number, 'p' represents the subshell type (dumbbell-shaped), and the '2' signifies the number of electrons. Importantly, the 2p subshell has three orbitals (2px, 2py, 2pz), each capable of holding two electrons. In carbon's ground state, these two electrons occupy separate 2p orbitals, following Hund's rule of maximum multiplicity (electrons fill orbitals individually before pairing up).
Orbital Hybridization: The Key to Carbon's Versatility
While the basic electronic configuration provides a foundational understanding, it doesn't fully explain carbon's ability to form a vast array of molecules with diverse geometries. This is where the concept of orbital hybridization comes into play. Orbital hybridization is a theoretical concept that describes the mixing of atomic orbitals within an atom to form new hybrid orbitals. This process significantly influences the geometry and bonding properties of molecules.
sp³ Hybridization: Tetrahedral Geometry
In many carbon compounds, the four valence electrons (the electrons in the outermost shell) participate in bonding. In this case, one 2s orbital and the three 2p orbitals hybridize to form four equivalent sp³ hybrid orbitals. These hybrid orbitals are arranged in a tetrahedral geometry, with bond angles of approximately 109.5°. This hybridization is responsible for the formation of molecules like methane (CH₄), where carbon forms four single bonds with four hydrogen atoms. The tetrahedral structure is a key factor in the three-dimensional nature of organic molecules.
sp² Hybridization: Trigonal Planar Geometry
When carbon forms double bonds, as in ethene (C₂H₄), sp² hybridization occurs. Here, one 2s orbital and two 2p orbitals hybridize to create three sp² hybrid orbitals arranged in a trigonal planar geometry with bond angles of approximately 120°. The remaining unhybridized 2p orbital participates in the formation of the pi (π) bond, which contributes to the double bond's characteristic properties.
sp Hybridization: Linear Geometry
In molecules containing triple bonds, such as ethyne (C₂H₂), sp hybridization takes place. One 2s orbital combines with one 2p orbital to produce two sp hybrid orbitals positioned linearly with a bond angle of 180°. The remaining two unhybridized 2p orbitals form two pi (π) bonds, resulting in the characteristic triple bond.
The Significance of Carbon's Electronic Configuration
The electronic configuration of carbon, along with its ability to undergo hybridization, is responsible for several key properties:
-
Tetravalency: Carbon's four valence electrons allow it to form four covalent bonds, contributing to the vast structural diversity of organic molecules. This tetravalency is crucial for the formation of long chains, branched structures, and rings, which are fundamental to the complexity of organic chemistry.
-
Catination: Carbon atoms can bond to other carbon atoms, forming chains and rings of virtually unlimited length. This property, known as catenation, is unique among elements and is a crucial factor in the existence of millions of organic compounds. Silicon exhibits some catenation, but it's significantly less extensive than carbon's.
-
Isomerism: The diverse ways in which carbon atoms can bond to each other and other elements lead to the possibility of isomers—molecules with the same molecular formula but different structural arrangements. This isomerism accounts for a vast number of distinct compounds with varying properties.
-
Bond Strength: The carbon-carbon bond is relatively strong, contributing to the stability of many organic molecules and enabling the formation of long-chain polymers.
-
Variety of Bond Types: Carbon readily forms single, double, and triple bonds, further expanding the range of possible molecular structures and properties.
Carbon's Role in Life
Carbon's unique electronic configuration and bonding capabilities are fundamental to life as we know it. The vast array of organic molecules—carbohydrates, lipids, proteins, and nucleic acids—all rely on carbon's ability to form complex structures. The intricate three-dimensional arrangements of atoms in these biomolecules are essential for their biological functions. DNA, the genetic blueprint of life, relies on a carbon backbone to store and transmit genetic information. Proteins, crucial for a myriad of cellular processes, also depend on carbon's ability to form complex chains and three-dimensional structures.
Advanced Concepts and Applications
The electronic configuration of carbon forms the basis for numerous advanced concepts in chemistry and related fields:
-
Molecular Orbital Theory: A more sophisticated approach to understanding bonding, molecular orbital theory considers the combination of atomic orbitals to form molecular orbitals that encompass the entire molecule. This approach provides a more accurate description of bonding in complex molecules.
-
Spectroscopy: Various spectroscopic techniques, such as NMR (Nuclear Magnetic Resonance) and IR (Infrared) spectroscopy, exploit the interactions between electromagnetic radiation and the electrons in molecules. Understanding carbon's electronic configuration is vital in interpreting spectroscopic data.
-
Computational Chemistry: Computer simulations and calculations are increasingly used to predict and understand the properties of molecules, including those containing carbon. Accurate models require a thorough understanding of electronic configurations.
-
Materials Science: The design and synthesis of new materials often involve manipulating carbon's bonding properties. Graphene, a single layer of carbon atoms arranged in a honeycomb lattice, exemplifies the potential of carbon-based materials. Its unique electronic and mechanical properties have led to extensive research and development in various applications. Similarly, carbon nanotubes, cylindrical structures of carbon atoms, possess exceptional strength and electrical conductivity.
Conclusion
The electronic configuration of carbon—1s²2s²2p²—is deceptively simple yet profoundly significant. Its four valence electrons, coupled with its ability to undergo hybridization, enable carbon to form an extraordinary range of molecules with diverse structures and properties. This versatility is the foundation of organic chemistry and is essential for the existence of life itself. The study of carbon's electronic configuration remains a cornerstone of scientific understanding, driving innovation in various fields, from materials science to medicine. Further exploration of its intricacies continues to reveal new possibilities and applications.
Latest Posts
Latest Posts
-
Limiting Reactant Practice Problems With Answers
Mar 21, 2025
-
What Is 1 6 Divided By 1 3
Mar 21, 2025
-
What Planets Are Smaller Than Earth
Mar 21, 2025
-
What Type Of Bonding Involves The Unequal Sharing Of Electrons
Mar 21, 2025
-
Where Is Most Of The Freshwater Found On Earth
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is Electronic Configuration Of Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
