What Type Of Bonding Involves The Unequal Sharing Of Electrons
listenit
Mar 21, 2025 · 7 min read
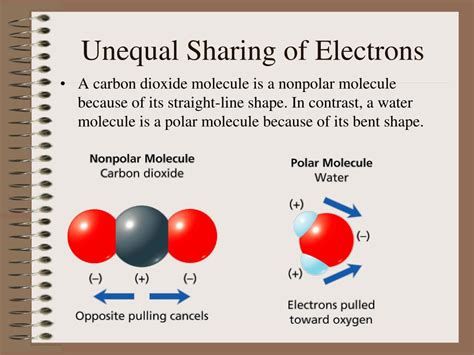
Table of Contents
What Type of Bonding Involves the Unequal Sharing of Electrons?
Polar covalent bonding is the type of bonding that involves the unequal sharing of electrons between atoms. This unequal sharing arises from differences in the electronegativity of the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. A large difference in electronegativity between two atoms leads to a polar covalent bond, while a small difference results in a nonpolar covalent bond, and a very large difference leads to an ionic bond. Understanding polar covalent bonding is crucial for comprehending the properties and behavior of a vast array of molecules and materials.
Understanding Electronegativity and its Role in Bonding
Before delving deeper into polar covalent bonds, it's essential to grasp the concept of electronegativity. This intrinsic property of an atom dictates how strongly it attracts electrons in a bond. Electronegativity values are generally presented on the Pauling scale, with fluorine (F) having the highest electronegativity value of 4.0. Elements on the right side of the periodic table, particularly nonmetals, generally exhibit higher electronegativity than elements on the left, primarily metals.
The electronegativity difference between two atoms participating in a bond directly impacts the nature of that bond.
-
Nonpolar Covalent Bond: When the electronegativity difference is small (typically less than 0.5), the electrons are shared almost equally between the atoms. This results in a nonpolar covalent bond, where the electron density is evenly distributed. Examples include bonds within diatomic molecules like O<sub>2</sub> and N<sub>2</sub>.
-
Polar Covalent Bond: When the electronegativity difference is moderate (typically between 0.5 and 1.7), the electrons are shared unequally. This unequal sharing creates a dipole moment, meaning one atom carries a partial negative charge (δ-) and the other carries a partial positive charge (δ+). This is a polar covalent bond. Examples include the O-H bond in water (H<sub>2</sub>O) and the C-O bond in carbon monoxide (CO).
-
Ionic Bond: When the electronegativity difference is very large (typically greater than 1.7), the more electronegative atom essentially steals the electron(s) from the less electronegative atom. This results in the formation of ions – positively charged cations and negatively charged anions – held together by electrostatic attraction. This is an ionic bond. Examples include NaCl (sodium chloride) and MgO (magnesium oxide).
Characteristics of Polar Covalent Bonds
Polar covalent bonds possess distinct characteristics that differentiate them from nonpolar covalent and ionic bonds:
-
Partial Charges: The unequal sharing of electrons leads to the development of partial positive (δ+) and partial negative (δ-) charges on the atoms involved. These are not full charges like in ionic bonds but represent a shift in electron density.
-
Dipole Moment: The separation of charge creates a dipole moment (µ), a vector quantity that represents the magnitude and direction of the bond's polarity. The dipole moment is calculated as the product of the charge separation and the distance between the charges.
-
Higher Boiling and Melting Points: Compared to nonpolar molecules of similar size, polar molecules generally exhibit higher boiling and melting points. This is because the dipole-dipole interactions between polar molecules are stronger than the weak London dispersion forces present in nonpolar molecules.
-
Solubility in Polar Solvents: Polar molecules tend to be soluble in polar solvents like water, due to the strong dipole-dipole interactions and hydrogen bonding (a special type of dipole-dipole interaction) that can occur.
-
Higher Reactivity: Polar covalent bonds often exhibit higher reactivity than nonpolar covalent bonds because the partial charges make them more susceptible to electrophilic and nucleophilic attacks.
Examples of Polar Covalent Bonds in Everyday Molecules
Many molecules essential for life and common in everyday applications contain polar covalent bonds:
-
Water (H₂O): The oxygen atom is significantly more electronegative than the hydrogen atoms, resulting in polar O-H bonds. This polarity is responsible for water's unique properties, including its high boiling point, surface tension, and ability to act as a universal solvent.
-
Carbon Dioxide (CO₂): While the molecule as a whole is linear and nonpolar, the individual C=O bonds are polar due to the electronegativity difference between carbon and oxygen. The symmetry of the molecule cancels out the individual dipole moments, resulting in a net zero dipole moment.
-
Glucose (C₆H₁₂O₆): This simple sugar contains numerous polar O-H and C-O bonds, making it highly soluble in water. This solubility is crucial for its role as an energy source in living organisms.
-
Ammonia (NH₃): The nitrogen atom is more electronegative than the hydrogen atoms, leading to polar N-H bonds. The pyramidal shape of the ammonia molecule results in a net dipole moment.
-
Hydrochloric Acid (HCl): The chlorine atom is significantly more electronegative than the hydrogen atom, resulting in a highly polar H-Cl bond. This high polarity contributes to HCl's strong acidity.
Polarity and Molecular Geometry
The overall polarity of a molecule depends not only on the polarity of its individual bonds but also on its molecular geometry. Even if a molecule contains polar bonds, the molecule might be nonpolar if the individual bond dipoles cancel each other out due to symmetry. For instance, carbon dioxide (CO₂) has polar C=O bonds, but its linear geometry causes the dipole moments to cancel, resulting in a nonpolar molecule. On the other hand, water (H₂O), with its bent geometry, has a net dipole moment because the bond dipoles don't cancel.
Consequences of Polar Covalent Bonding
The presence of polar covalent bonds significantly impacts the physical and chemical properties of substances. The partial charges created by unequal electron sharing lead to:
-
Dipole-dipole interactions: Attractive forces between the positive end of one polar molecule and the negative end of another. These forces are stronger than London dispersion forces, leading to higher melting and boiling points.
-
Hydrogen bonding: A special type of dipole-dipole interaction that occurs when hydrogen is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine). Hydrogen bonds are relatively strong and contribute significantly to the properties of water and many biological molecules.
-
Increased solubility in polar solvents: Polar molecules readily dissolve in polar solvents because of the attractive forces between the solute and solvent molecules.
Differentiating Polar Covalent from Ionic Bonding
While both polar covalent and ionic bonds involve unequal sharing of electrons, the degree of this inequality distinguishes them. In polar covalent bonds, the electrons are shared unequally, resulting in partial charges. In ionic bonds, the electrons are essentially transferred, creating fully charged ions. The electronegativity difference is the key factor: a large difference leads to ionic bonding, while a moderate difference leads to polar covalent bonding. The boundary between these two bond types isn't always sharply defined, and some bonds exhibit characteristics of both.
Applications and Significance of Polar Covalent Bonds
Polar covalent bonds are ubiquitous in chemistry and biology. Their understanding is fundamental to:
-
Drug design: The polarity of molecules is critical for their interactions with biological targets, influencing drug absorption, distribution, metabolism, and excretion.
-
Material science: The properties of many materials, including polymers, ceramics, and semiconductors, are directly related to the nature of the bonds within them.
-
Environmental science: The polarity of molecules influences their behavior in the environment, including their solubility, mobility, and reactivity.
-
Biochemistry: The vast majority of biological molecules, including proteins, carbohydrates, and nucleic acids, are held together by polar covalent bonds.
Conclusion
Polar covalent bonding, driven by the unequal sharing of electrons due to differences in electronegativity, is a fundamental concept in chemistry. This type of bonding leads to the creation of molecules with unique properties and plays a vital role in a wide array of chemical and biological processes. Understanding the principles of electronegativity and molecular geometry is crucial for predicting and explaining the behavior of molecules and materials. The impact of polar covalent bonds extends across various scientific disciplines, highlighting its significance in our understanding of the natural world and its applications in technological advancements.
Latest Posts
Latest Posts
-
How To Write 40 As A Fraction
Mar 22, 2025
-
How Many Punts In A Quart
Mar 22, 2025
-
What Is The Shape Of A Planetary Orbit
Mar 22, 2025
-
How Much Is A Half Mile
Mar 22, 2025
-
What Is 9 Oz In Cups
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bonding Involves The Unequal Sharing Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
