What Is A Polymer Of Amino Acids
listenit
Mar 31, 2025 · 7 min read
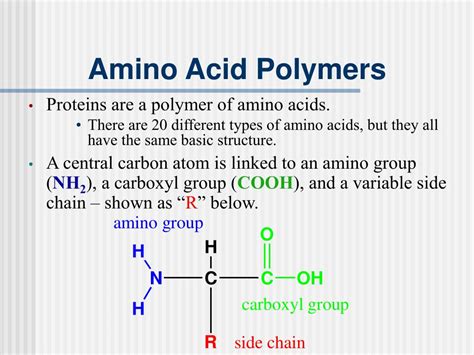
Table of Contents
What is a Polymer of Amino Acids? Understanding Proteins and Their Functions
Proteins are the workhorses of life, performing a vast array of functions crucial for the survival and proper functioning of all living organisms. From catalyzing biochemical reactions to providing structural support, proteins are essential components of cells and tissues. But what exactly are proteins? At their core, proteins are polymers of amino acids. This seemingly simple statement encapsulates a world of complexity and intricacy, a world we will explore in detail in this article.
Understanding Amino Acids: The Building Blocks of Proteins
Before delving into the intricacies of protein structure, let's first understand the fundamental building blocks: amino acids. These organic molecules are characterized by a central carbon atom (the alpha carbon) bonded to four groups:
- An amino group (-NH₂): This group is basic, meaning it can accept a proton (H⁺).
- A carboxyl group (-COOH): This group is acidic, meaning it can donate a proton (H⁺).
- A hydrogen atom (-H): A simple hydrogen atom.
- A side chain (R-group): This is the variable group that distinguishes one amino acid from another. The R-group can be anything from a simple hydrogen atom (as in glycine) to a complex aromatic ring structure (as in tryptophan). The properties of the R-group (size, charge, polarity, etc.) significantly influence the overall properties and function of the protein.
There are 20 standard amino acids that are commonly found in proteins. These amino acids are encoded by the genetic code and are used by ribosomes to synthesize proteins during translation. While there are other amino acids present in nature, these 20 form the basis of protein structure and function.
Properties of Amino Acids and Their Impact on Protein Structure
The diverse chemical properties of the 20 standard amino acids are crucial in determining the three-dimensional structure of a protein. These properties can be broadly categorized as follows:
-
Hydrophobic (nonpolar): These amino acids have nonpolar side chains and tend to cluster together in the interior of a protein, away from the aqueous environment of the cell. Examples include alanine, valine, leucine, isoleucine, methionine, phenylalanine, tryptophan, and proline.
-
Hydrophilic (polar): These amino acids have polar side chains and are often found on the surface of a protein, interacting with the surrounding water molecules. Examples include serine, threonine, cysteine, tyrosine, asparagine, and glutamine.
-
Charged: These amino acids possess charged side chains, either positively or negatively charged. These charges play a vital role in protein-protein interactions and enzymatic activity. Examples of positively charged amino acids (basic) include lysine, arginine, and histidine. Examples of negatively charged amino acids (acidic) include aspartic acid and glutamic acid.
The Polymerization of Amino Acids: Peptide Bonds and Primary Structure
Amino acids link together to form proteins through a process called peptide bond formation. This is a dehydration reaction, meaning a water molecule is removed during the bond formation. The carboxyl group (-COOH) of one amino acid reacts with the amino group (-NH₂) of another amino acid, forming a peptide bond (-CO-NH-) and releasing a water molecule (H₂O).
The resulting chain of amino acids is called a polypeptide. The sequence of amino acids in a polypeptide chain is known as its primary structure. This sequence is determined by the genetic code and is crucial in determining the higher-order structures and, ultimately, the function of the protein. Even a single amino acid change can drastically alter the protein's function, as seen in many genetic diseases.
Primary Structure and its Importance
The primary structure is not just a random sequence of amino acids. It is dictated by the specific gene that codes for that particular protein. This sequence dictates how the polypeptide chain will fold into its three-dimensional structure and, therefore, its function. Mutations in the gene can lead to changes in the amino acid sequence, resulting in non-functional or misfolded proteins. These misfolded proteins can be the cause of numerous diseases.
Beyond the Primary Structure: Secondary, Tertiary, and Quaternary Structures
The primary structure of a protein dictates its higher-order structures, which are critical for its biological activity. These higher-order structures are stabilized by various non-covalent interactions, including:
-
Hydrogen bonds: These relatively weak bonds play a crucial role in stabilizing secondary structures like alpha-helices and beta-sheets.
-
Hydrophobic interactions: The clustering of hydrophobic amino acid side chains in the protein's core contributes to the protein's overall stability.
-
Ionic interactions (salt bridges): These interactions occur between oppositely charged amino acid side chains.
-
Disulfide bonds: These covalent bonds between cysteine residues are stronger than non-covalent interactions and contribute significantly to the protein's stability.
Secondary Structure: Alpha-Helices and Beta-Sheets
The polypeptide chain doesn't remain as a linear structure. It folds into regular repeating structures known as secondary structures. The two most common secondary structures are:
-
Alpha-helices: These are coiled structures stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues away.
-
Beta-sheets: These are formed by hydrogen bonds between adjacent polypeptide chains or segments of the same polypeptide chain that are arranged in a parallel or antiparallel manner.
Tertiary Structure: The Three-Dimensional Arrangement
The overall three-dimensional arrangement of a polypeptide chain, including its secondary structures, is referred to as its tertiary structure. This structure is determined by the interactions between amino acid side chains, as well as the interactions between different secondary structures. The tertiary structure is crucial for the protein's function as it dictates the arrangement of active sites in enzymes or binding sites in receptor proteins.
Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these individual polypeptide chains, also known as subunits, is called the quaternary structure. Hemoglobin, the oxygen-carrying protein in red blood cells, is a classic example of a protein with a quaternary structure, comprising four subunits.
Protein Function: A Diverse Array of Roles
The diverse structures of proteins directly correlate with their incredibly diverse functions. Some key functions include:
-
Enzymes: These are biological catalysts that speed up chemical reactions in the cell. Examples include digestive enzymes like amylase and protease.
-
Structural proteins: These proteins provide support and shape to cells and tissues. Collagen and elastin are examples of structural proteins found in connective tissues.
-
Transport proteins: These proteins facilitate the movement of molecules across cell membranes. Hemoglobin and membrane channels are examples of transport proteins.
-
Motor proteins: These proteins generate movement, such as muscle contraction (myosin and actin) and intracellular transport.
-
Hormones: These proteins act as chemical messengers, coordinating various cellular activities. Insulin and growth hormone are examples of protein hormones.
-
Antibodies: These proteins play a crucial role in the immune system, recognizing and neutralizing foreign invaders.
-
Receptors: These proteins bind to specific molecules, triggering intracellular signaling pathways.
The Importance of Protein Folding and Misfolding
The proper folding of a protein is essential for its function. The process of protein folding is complex and involves a delicate balance of interactions between amino acid side chains. However, under certain conditions, proteins can misfold, leading to the formation of aggregates that can be detrimental to the cell. Misfolded proteins are implicated in various diseases, including Alzheimer's disease, Parkinson's disease, and cystic fibrosis.
Conclusion: The Intricate World of Protein Polymers
Proteins, polymers of amino acids, are essential molecules of life, playing crucial roles in virtually every biological process. Understanding the intricate relationship between the amino acid sequence (primary structure), the higher-order structures, and the ultimate function of a protein is critical to understanding biology at a fundamental level. The diverse properties of amino acids and the various types of interactions that stabilize protein structures contribute to the incredible diversity and functionality of these vital biomolecules. Further research continues to unravel the complex mechanisms of protein folding, misfolding, and their implications for human health, opening new avenues for therapeutic interventions and disease prevention.
Latest Posts
Latest Posts
-
What Is 24 Divided By 4
Apr 01, 2025
-
Milk Turning Sour Is A Chemical Change
Apr 01, 2025
-
Where On The Periodic Table Are Metals Found
Apr 01, 2025
-
What Is 0 875 In A Fraction
Apr 01, 2025
-
How To Find The Secant Line
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is A Polymer Of Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
