What Happens When An Ionic Compound Dissolves In Water
listenit
Mar 20, 2025 · 5 min read
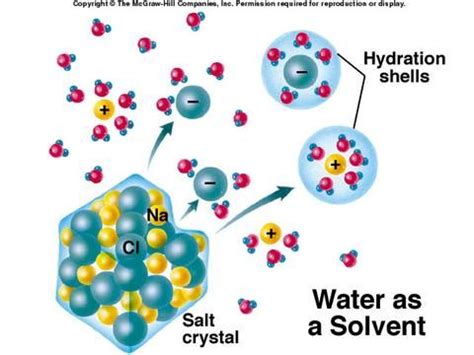
Table of Contents
What Happens When an Ionic Compound Dissolves in Water?
Understanding what happens when an ionic compound dissolves in water is fundamental to chemistry. It's a process driven by the powerful forces of attraction and repulsion between molecules and ions, ultimately affecting various aspects of our daily lives, from the way we clean to the functioning of our bodies. This comprehensive article dives deep into the intricacies of this phenomenon, exploring the underlying principles, the role of water's unique properties, and the implications of this process in different contexts.
The Nature of Ionic Compounds
Before we delve into the dissolution process, it's essential to understand the nature of ionic compounds themselves. These compounds are formed through the electrostatic attraction between oppositely charged ions: cations (positively charged ions) and anions (negatively charged ions). This strong attraction results in a crystalline structure where ions are arranged in a highly ordered, three-dimensional lattice. Common examples of ionic compounds include sodium chloride (NaCl, table salt), potassium iodide (KI), and calcium carbonate (CaCO₃).
The strength of the ionic bond depends on several factors, most importantly the charge of the ions and the distance between them. Higher charges and smaller distances lead to stronger bonds, making the compound more difficult to dissolve.
The Unique Properties of Water
Water, as the universal solvent, plays a crucial role in the dissolution of ionic compounds. Its remarkable ability stems from its polar nature. The oxygen atom in a water molecule (H₂O) is more electronegative than the hydrogen atoms, resulting in a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This uneven distribution of charge makes water a polar molecule, capable of interacting with ions through dipole-dipole interactions.
Hydrogen Bonding: A Key Player
Water molecules also exhibit strong hydrogen bonding, an intermolecular force stronger than typical dipole-dipole interactions. This arises from the strong attraction between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another. This extensive hydrogen bonding network contributes significantly to water's high boiling point, surface tension, and its exceptional solvent properties.
The Dissolution Process: A Step-by-Step Look
The dissolution of an ionic compound in water is a dynamic equilibrium process involving several key steps:
1. Ion-Dipole Interactions: The Initial Attraction
When an ionic compound is added to water, the polar water molecules are attracted to the charged ions on the surface of the crystal lattice. The partially positive hydrogen atoms in water molecules are drawn to the anions, while the partially negative oxygen atoms are attracted to the cations. This attraction is known as ion-dipole interaction.
These interactions weaken the electrostatic forces holding the ions together in the crystal lattice. The stronger the ion-dipole interactions, the more effective water is at dissolving the ionic compound.
2. Hydration: Surrounding Ions with Water Molecules
As the ion-dipole interactions increase, water molecules begin to surround individual ions, a process called hydration. Each ion becomes surrounded by a shell of water molecules, effectively shielding it from the other ions in the lattice. This hydration shell stabilizes the ions in solution and prevents them from recombining to form the crystal lattice. The energy released during hydration is called the hydration enthalpy.
The size and charge of the ion significantly influence the strength of hydration. Smaller, highly charged ions have stronger hydration shells, making them more soluble.
3. Breaking the Lattice: Overcoming Electrostatic Forces
The energy released through hydration must be sufficient to overcome the strong electrostatic forces holding the ions together in the crystal lattice. This lattice energy is a measure of the strength of the ionic bonds. If the hydration enthalpy is greater than the lattice energy, the ionic compound will dissolve. If not, the compound remains undissolved.
4. Dispersion of Ions: Formation of a Solution
Once the ions are separated and surrounded by water molecules, they disperse throughout the solution, becoming uniformly distributed. This forms an aqueous solution containing hydrated ions. The solution is now electrically neutral as the positive and negative charges of the dissolved ions balance each other out.
Factors Affecting Solubility
Several factors can influence the solubility of an ionic compound in water:
- Lattice energy: Higher lattice energy implies stronger ionic bonds, reducing solubility.
- Hydration enthalpy: Higher hydration enthalpy favors dissolution.
- Temperature: Increased temperature generally increases solubility, as it provides more kinetic energy to overcome the lattice energy.
- Pressure: Pressure has a negligible effect on the solubility of ionic solids in water.
- Common ion effect: The presence of a common ion in the solution reduces the solubility of the ionic compound.
- pH: The pH of the solution can affect the solubility of certain ionic compounds, especially those involving weak acids or bases.
Applications and Implications
The dissolution of ionic compounds in water has numerous practical applications and implications:
- Biological systems: Many biological processes rely on the dissolution of ionic compounds. For example, the transport of ions across cell membranes is crucial for nerve impulse transmission and muscle contraction. Electrolytes in our bodies, like sodium and potassium ions, are essential for maintaining proper fluid balance and nerve function.
- Industrial processes: Many industrial processes rely on the solubility of ionic compounds. For example, the production of chemicals, fertilizers, and pharmaceuticals often involves dissolving ionic compounds in water.
- Environmental science: Understanding the solubility of ionic compounds is critical for assessing water quality and environmental contamination. The solubility of pollutants can affect their transport and bioavailability in the environment.
- Medicine: Many drugs are administered as aqueous solutions of ionic compounds. Solubility is crucial for drug absorption and effectiveness.
Conclusion
The dissolution of an ionic compound in water is a complex but fascinating process governed by a delicate balance between electrostatic forces and hydration energies. It’s a dynamic equilibrium where the unique properties of water play a crucial role. This fundamental chemical process underlies countless phenomena, from everyday processes to sophisticated biological and industrial applications. A thorough understanding of this process is crucial across various scientific disciplines and is vital in many practical applications. Further research continues to refine our understanding of this process, leading to improvements in various fields and further advancements in science and technology. The seemingly simple act of salt dissolving in water highlights the complex interplay of forces and interactions at the molecular level that shape our world.
Latest Posts
Latest Posts
-
How To Find All Zeros In A Function
Mar 21, 2025
-
X 3 5x 2 X 5
Mar 21, 2025
-
How To Calculate Heat Of Neutralization
Mar 21, 2025
-
How To Find The Perpendicular Vector
Mar 21, 2025
-
Symbol For Aluminum On Periodic Table
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Happens When An Ionic Compound Dissolves In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
