How To Calculate Heat Of Neutralization
listenit
Mar 21, 2025 · 6 min read
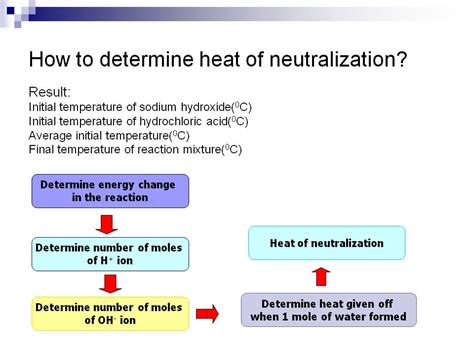
Table of Contents
How to Calculate the Heat of Neutralization: A Comprehensive Guide
The heat of neutralization, also known as the enthalpy of neutralization, is the heat change that occurs when one equivalent of an acid reacts completely with one equivalent of a base in dilute solution. This process is an exothermic reaction, meaning it releases heat into the surroundings, causing a temperature increase. Understanding how to calculate this heat change is crucial in various fields, including chemistry, chemical engineering, and environmental science. This comprehensive guide will walk you through the process, explaining the concepts, calculations, and potential sources of error.
Understanding the Fundamentals
Before diving into the calculations, let's solidify our understanding of the underlying principles:
What is Enthalpy?
Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. The change in enthalpy (ΔH) during a reaction reflects the heat transferred between the system and its surroundings. In exothermic reactions, ΔH is negative (heat is released), while in endothermic reactions, ΔH is positive (heat is absorbed).
The Neutralization Reaction
Neutralization reactions involve the reaction between an acid and a base, resulting in the formation of salt and water. A typical example is the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
The heat released during this reaction is the heat of neutralization. The magnitude of this heat depends on the strength of the acid and base involved. Strong acids and strong bases usually release more heat than weak acids and weak bases.
Calorimetry: Measuring Heat Transfer
The heat of neutralization is typically determined experimentally using calorimetry. A calorimeter is a device used to measure heat changes in a chemical reaction. A simple calorimeter might consist of a polystyrene cup (to minimize heat loss) containing the acid solution, into which the base solution is added. The temperature change is monitored using a thermometer.
Calculating the Heat of Neutralization: A Step-by-Step Approach
The calculation involves several key steps:
1. Determine the Temperature Change (ΔT):
The most crucial step is accurately measuring the temperature change during the reaction. Record the initial temperature (Tᵢ) of the acid solution before adding the base and the final temperature (Tƒ) after the reaction has reached equilibrium. The temperature change is:
ΔT = Tƒ - Tᵢ
2. Calculate the Heat Absorbed by the Solution (q<sub>solution</sub>):
The heat absorbed by the solution (q<sub>solution</sub>) can be calculated using the following formula:
q<sub>solution</sub> = m × c × ΔT
Where:
- m is the total mass of the solution (in grams). This is the sum of the masses of the acid and base solutions.
- c is the specific heat capacity of the solution (usually approximated to be the same as water, 4.18 J/g°C).
- ΔT is the temperature change (calculated in step 1).
3. Account for Heat Loss (q<sub>loss</sub>):
In reality, some heat will be lost to the surroundings. While a polystyrene cup minimizes this loss, it's not completely eliminated. Advanced calorimetry techniques address this more precisely; however, for simpler experiments, this loss is often neglected or approximated.
4. Calculate the Heat Released by the Reaction (q<sub>reaction</sub>):
The heat released by the neutralization reaction (q<sub>reaction</sub>) is equal in magnitude but opposite in sign to the heat absorbed by the solution (assuming minimal heat loss):
q<sub>reaction</sub> = -q<sub>solution</sub>
5. Determine the Number of Moles (n):
Calculate the number of moles of the limiting reactant (the reactant that is completely consumed in the reaction) using its molar mass and the volume used in the experiment. For example, if using 100 ml of 1M HCl, you have 0.1 moles of HCl.
6. Calculate the Heat of Neutralization (ΔH<sub>neutralization</sub>):
Finally, calculate the heat of neutralization using the following formula:
ΔH<sub>neutralization</sub> = q<sub>reaction</sub> / n
This will give the enthalpy change (ΔH) in Joules per mole (J/mol). You can convert it to kilojoules per mole (kJ/mol) by dividing by 1000.
Example Calculation
Let's work through a hypothetical example:
50.0 ml of 1.0 M HCl is mixed with 50.0 ml of 1.0 M NaOH in a calorimeter. The initial temperature is 25.0 °C, and the final temperature is 31.5 °C. Assume the specific heat capacity of the solution is 4.18 J/g°C, and the density of the solution is approximately 1 g/ml.
-
ΔT = 31.5 °C - 25.0 °C = 6.5 °C
-
m = (50.0 ml + 50.0 ml) × 1 g/ml = 100 g
-
q<sub>solution</sub> = 100 g × 4.18 J/g°C × 6.5 °C = 2717 J
-
q<sub>reaction</sub> = -2717 J
-
Moles of HCl (limiting reactant): 0.05 L × 1.0 mol/L = 0.05 mol
-
ΔH<sub>neutralization</sub> = -2717 J / 0.05 mol = -54340 J/mol = -54.3 kJ/mol
Therefore, the heat of neutralization for this reaction is approximately -54.3 kJ/mol. The negative sign indicates an exothermic reaction.
Sources of Error and Improvements
Several factors can affect the accuracy of the calculated heat of neutralization:
- Heat Loss: Heat loss to the surroundings is a major source of error. Using a more sophisticated calorimeter (like a coffee-cup calorimeter with a lid or a bomb calorimeter) can minimize this.
- Incomplete Reaction: If the acid and base do not react completely, the calculated heat of neutralization will be lower than the actual value. Using an excess of one reactant can help ensure complete reaction.
- Specific Heat Capacity: The assumption that the specific heat capacity of the solution is the same as water may not always be accurate. Using a more precise value for the specific heat capacity of the resulting solution improves accuracy.
- Heat Capacity of the Calorimeter: The calorimeter itself absorbs some heat during the reaction. A more accurate calculation should consider the heat capacity of the calorimeter.
- Mixing Time: It takes time for the solution to achieve an equilibrium temperature. Ensure adequate time for mixing and temperature stabilization before recording final temperature.
Advanced Considerations
For more accurate results, consider the following:
- Using a Coffee-Cup Calorimeter: A coffee-cup calorimeter provides better insulation than a simple polystyrene cup, reducing heat loss to the environment.
- Correction for Heat Capacity of the Calorimeter: Include the heat capacity of the calorimeter itself in the calculation to account for the heat absorbed by the apparatus.
- Using a Bomb Calorimeter: For higher accuracy, a bomb calorimeter, which operates under constant volume, is used. This is particularly helpful for reactions involving gases.
Conclusion
Calculating the heat of neutralization requires careful experimental procedures and accurate calculations. By following the steps outlined in this guide and considering the potential sources of error, you can obtain a reliable measure of this important thermodynamic property. Remember to always prioritize safety when conducting experiments involving chemicals. This detailed explanation provides a solid foundation for understanding and performing these crucial calculations in various chemical contexts. Further research into advanced calorimetry techniques can lead to more precise and refined results.
Latest Posts
Latest Posts
-
A Conical Tank With Vertex Down
Mar 22, 2025
-
What Is 60 Percent Of 25
Mar 22, 2025
-
How Do You Write 1 9 As A Decimal
Mar 22, 2025
-
Which Is The Most Reactive Metal
Mar 22, 2025
-
1 1 4 As A Whole Number
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Heat Of Neutralization . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
