What Element Is Found In Proteins
listenit
Mar 26, 2025 · 6 min read
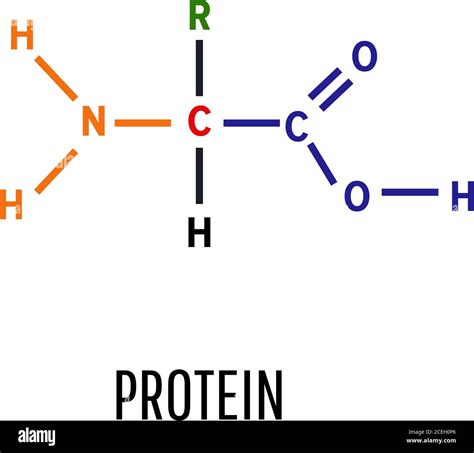
Table of Contents
What Element is Found in Proteins? A Deep Dive into Protein Composition
Proteins are the workhorses of life, essential for virtually every biological process. Understanding their composition is key to understanding their function. While the answer to the question "What element is found in proteins?" might seem simple at first glance, delving deeper reveals a fascinating complexity. This article will explore not just the primary element, but the intricate interplay of elements that contribute to the diverse structures and functions of proteins.
The Primary Element: Carbon
The most abundant element found in proteins is carbon (C). Carbon's unique ability to form four strong covalent bonds makes it the ideal backbone for the long chains of amino acids that constitute proteins. These chains, also known as polypeptide chains, are formed through peptide bonds, which are covalent bonds between the carboxyl group of one amino acid and the amino group of another. This carbon backbone provides the structural framework for the protein's three-dimensional shape, which is crucial for its function.
Carbon's Role in Amino Acid Structure
Each amino acid, the building block of a protein, contains a central carbon atom, often referred to as the alpha-carbon. This alpha-carbon is bonded to four different groups:
- An amino group (-NH2): This group is responsible for the basic properties of amino acids.
- A carboxyl group (-COOH): This group contributes acidic properties to amino acids.
- A hydrogen atom (H): This simple atom contributes to the overall structure.
- A side chain (R group): This variable group is what distinguishes the 20 different standard amino acids. The R group's properties (e.g., size, charge, polarity) significantly impact the protein's overall structure and function.
The diversity in R groups, and therefore amino acids, arises from the different ways carbon atoms can bond with other atoms, creating a vast array of chemical functionalities. This explains the incredible diversity and functional versatility of proteins.
Other Essential Elements: Hydrogen, Oxygen, and Nitrogen
Besides carbon, proteins contain significant amounts of hydrogen (H), oxygen (O), and nitrogen (N). These elements are integral components of the amino acid structure and contribute to the protein's overall chemical properties.
Hydrogen's Role in Protein Structure and Function
Hydrogen atoms are abundant in proteins, forming part of the amino and carboxyl groups in the amino acid backbone, as well as in the side chains of many amino acids. They play a crucial role in hydrogen bonding, a weak yet vital intermolecular force that helps stabilize the protein's three-dimensional structure. Hydrogen bonds contribute to the formation of secondary structures like alpha-helices and beta-sheets, and also play a role in tertiary and quaternary structure stabilization.
Oxygen's Contribution
Oxygen is found in the carboxyl group of each amino acid and is also present in the side chains of certain amino acids like serine, threonine, and tyrosine. Oxygen's electronegativity contributes to the polarity of certain amino acids and influences their interactions within the protein structure. It also participates in various chemical reactions catalyzed by enzymes, which are proteins.
Nitrogen's Significance
Nitrogen is a vital element in proteins, forming part of the amino group of each amino acid. This amino group is crucial for the formation of peptide bonds, linking amino acids together to form polypeptide chains. Nitrogen's presence is essential for the protein's overall structure and its ability to function. Nitrogen-containing side chains in certain amino acids, like lysine and arginine, also play critical roles in protein function, often involving interactions with other molecules.
Minor Elements: Sulfur, Phosphorus, and Metals
While carbon, hydrogen, oxygen, and nitrogen are the major elements, several other elements occur in smaller quantities but still play significant roles in specific proteins.
Sulfur's Role in Disulfide Bonds
Sulfur (S) is found in the side chains of the amino acids cysteine and methionine. Cysteine residues can form disulfide bonds, a type of covalent bond that contributes significantly to the stability of the protein's three-dimensional structure, particularly in proteins secreted outside the cell. These disulfide bonds act as strong cross-links, preventing unfolding and maintaining the protein's functionality.
Phosphorus's Importance in Phosphorylation
Phosphorus (P) is found in some proteins, typically through the addition of phosphate groups during post-translational modifications. Phosphorylation, the addition of a phosphate group, is a crucial regulatory mechanism in many cellular processes. It alters the protein's conformation and activity, acting as an on/off switch for enzyme function.
Metal Ions: Essential Cofactors
Many proteins require metal ions as cofactors for their catalytic activity. These metal ions, such as iron (Fe), zinc (Zn), copper (Cu), magnesium (Mg), and calcium (Ca), bind to specific sites within the protein structure and play critical roles in enzyme function. For instance, iron is essential for the oxygen-carrying protein hemoglobin, while zinc is a structural component of many enzymes.
The Interplay of Elements: Determining Protein Structure and Function
The precise arrangement and interaction of these elements within a protein molecule dictate its three-dimensional structure and, consequently, its function. The combination and proportion of these elements influence factors such as:
- Solubility: The presence of polar and nonpolar side chains, determined by the constituent elements in the amino acids, dictates a protein's solubility in water.
- Charge: The amino acid side chains containing nitrogen and oxygen atoms influence the overall charge of the protein, affecting its interactions with other molecules.
- Stability: Disulfide bonds (sulfur) and hydrogen bonds (hydrogen and oxygen) contribute to a protein’s stability.
- Catalytic Activity: Metal ions act as cofactors in many enzymes, playing a crucial role in their catalytic activity.
- Protein-Protein Interactions: The charge distribution and presence of specific functional groups, determined by the constituent elements, influence a protein’s ability to interact with other proteins.
Beyond the Basic Elements: Isotopes and Trace Elements
While the elements mentioned above are the major components, it's important to note that isotopes of these elements, as well as trace elements, can also be present in proteins. Isotopes are atoms of the same element with differing numbers of neutrons. These variations can be used in research techniques, such as radioactive labeling, to track protein synthesis and movement within cells. Trace elements, present in extremely small quantities, might have specific roles in certain proteins, though their functions are often less well-understood.
Conclusion: A Complex Collaboration
The answer to "What element is found in proteins?" is far more nuanced than simply "carbon." Proteins are complex macromolecules composed of a collaboration of elements, each playing a vital role in shaping their structure, function, and interactions within living organisms. Understanding the precise interplay of these elements—carbon, hydrogen, oxygen, nitrogen, sulfur, phosphorus, and various metal ions—is crucial for comprehending the remarkable diversity and essential roles proteins play in all aspects of life. Further research continually uncovers new intricacies in protein composition and function, highlighting the enduring complexity of these remarkable biological molecules.
Latest Posts
Latest Posts
-
Do Parallelograms Have 4 Right Angles
Mar 29, 2025
-
Least Common Multiple Of 10 And 8
Mar 29, 2025
-
Name 3 Ways To Dissolve Something Faster
Mar 29, 2025
-
Charged Language In I Have A Dream
Mar 29, 2025
-
What Percentage Is 38 Out Of 40
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Element Is Found In Proteins . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
