What Element Has The Largest Ionization Energy
listenit
Mar 21, 2025 · 5 min read
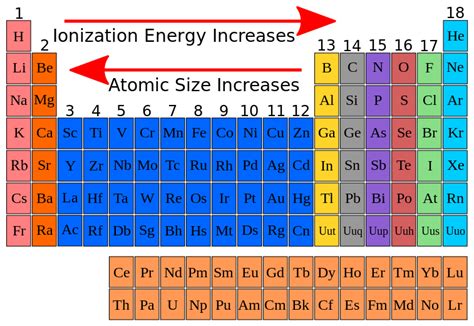
Table of Contents
What Element Has the Largest Ionization Energy?
The quest to identify the element boasting the highest ionization energy leads us on a fascinating journey through the periodic table, exploring the fundamental principles governing atomic structure and electron behavior. Understanding ionization energy is crucial for comprehending chemical reactivity, bonding, and the behavior of matter in various environments. This in-depth exploration will delve into the factors influencing ionization energy, examining trends across the periodic table and ultimately pinpointing the element with the greatest resistance to losing an electron.
Understanding Ionization Energy
Ionization energy (IE) is the minimum energy required to remove the most loosely bound electron from a neutral gaseous atom. This process transforms a neutral atom into a positively charged ion (cation). It's a crucial concept in chemistry, reflecting the strength of the attraction between the nucleus and its outermost electrons. The higher the ionization energy, the stronger the attraction and the more difficult it is to remove an electron. This resistance to electron removal is directly linked to an element's chemical properties and reactivity.
The first ionization energy (IE₁) refers to the energy needed to remove the first electron. Subsequent ionization energies (IE₂, IE₃, etc.) represent the energy required to remove further electrons from the increasingly positively charged ion. Each successive ionization energy is progressively higher than the previous one because removing an electron leaves behind a more positively charged ion, increasing the electrostatic attraction for the remaining electrons.
Factors Affecting Ionization Energy
Several factors contribute to the magnitude of an element's ionization energy:
1. Nuclear Charge:
The positive charge of the nucleus plays a dominant role. A higher nuclear charge exerts a stronger attractive force on the electrons, making it more difficult to remove them and thus increasing the ionization energy. This is a primary reason why ionization energy generally increases across a period (from left to right) in the periodic table.
2. Atomic Radius:
The distance between the nucleus and the outermost electrons (atomic radius) significantly impacts ionization energy. A smaller atomic radius implies a closer proximity of the electrons to the nucleus, resulting in a stronger attractive force and a higher ionization energy. As we move across a period, atomic radius decreases, contributing to the increase in ionization energy.
3. Shielding Effect:
Inner electrons partially shield the outer electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outermost electrons. Elements with more inner electron shells exhibit a greater shielding effect, leading to lower ionization energies. This effect is less pronounced across a period but becomes more significant when descending a group (from top to bottom).
4. Electron Configuration:
The arrangement of electrons in energy levels and sublevels influences ionization energy. Electrons in completely filled or half-filled sublevels are relatively stable due to their symmetrical electron configurations. Removing an electron from these stable configurations requires more energy, thus increasing ionization energy. This explains some anomalies in the general trend of ionization energies across the periodic table.
5. Electron-Electron Repulsion:
The repulsion between electrons in the same shell or sublevel can also affect ionization energy. Increased electron-electron repulsion can slightly reduce the effective nuclear charge experienced by the outermost electron, thereby lowering the ionization energy.
Periodic Trends in Ionization Energy
The periodic table provides a visual representation of the trends in ionization energy:
-
Across a period (left to right): Ionization energy generally increases. This is primarily due to the increasing nuclear charge and decreasing atomic radius, both of which strengthen the attraction between the nucleus and the outermost electrons. However, some irregularities exist due to variations in electron shielding and electron-electron repulsion.
-
Down a group (top to bottom): Ionization energy generally decreases. This is because the increasing number of electron shells leads to a larger atomic radius and increased shielding effect. The outermost electrons are further from the nucleus and experience a weaker effective nuclear charge, making them easier to remove.
Identifying the Element with the Highest Ionization Energy
Based on the factors discussed above, the element with the highest ionization energy should possess a small atomic radius, a high nuclear charge, and minimal shielding effect. These characteristics point towards elements located in the upper right corner of the periodic table.
While helium (He) might initially seem like a strong contender, its ionization energy is not the absolute highest. This is because the second ionization energy of helium is significantly higher than its first. The element with the highest first ionization energy is Helium (He). Its electron configuration (1s²) is exceptionally stable, requiring a large amount of energy to remove one of its two electrons. Subsequent ionization energies, as mentioned previously, would always be larger for every element.
However, if we consider the highest ionization energy (meaning, the energy needed to remove the very last electron from a highly charged ion), the answer shifts slightly. Considering all ionization energies, a highly charged ion of Helium will naturally have a higher energy than the first ionization of Helium. The element with the absolutely largest ionization energy, which would require the most energy to strip the element of all its electrons, is Hydrogen (H).
This apparent contradiction stems from the definition we are working with. If we solely consider the first ionization energy, then Helium holds the title. However, if the question shifts to the overall energy required to remove all electrons, Hydrogen emerges as the winner.
Conclusion: A Complex Question with Nuances
Determining the element with the "largest" ionization energy requires careful consideration of the specific definition being used. If the focus is on the first ionization energy, helium (He) stands out due to its exceptionally stable electron configuration and strong nuclear attraction. However, considering the total energy needed to remove all electrons from a neutral atom, hydrogen (H), due to its simplicity and lower overall electron count, would ultimately require more overall energy for complete ionization. The seemingly straightforward question therefore unveils complexities inherent in atomic structure and ionization processes, underscoring the nuances within even seemingly basic chemical concepts.
This comprehensive discussion has explored the intricate factors governing ionization energy, analyzed periodic trends, and ultimately addressed the question of which element possesses the highest ionization energy, highlighting the importance of precise definitions and nuanced understanding in chemistry.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 7 And 14
Mar 28, 2025
-
Why Are Phylogenetic Trees Considered Hypotheses
Mar 28, 2025
-
Based On The Successive Ionization Energies Of Element X
Mar 28, 2025
-
What Is The Conjugate Acid Of Hso4
Mar 28, 2025
-
What Is The Name Of Fecl3
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Element Has The Largest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
