What Element Has 4 Valence Electrons
listenit
Mar 30, 2025 · 6 min read
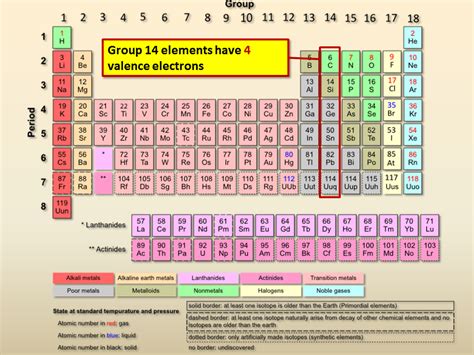
Table of Contents
What Element Has 4 Valence Electrons? Exploring Group 14 Elements
The question "What element has 4 valence electrons?" leads us into the fascinating world of chemistry and the periodic table. The answer isn't a single element, but rather a whole group of elements sharing a key characteristic: four valence electrons. These elements, found in Group 14 (formerly IVA) of the periodic table, exhibit unique properties and play vital roles in various applications. This article delves deep into the properties, characteristics, and applications of these fascinating elements.
Understanding Valence Electrons
Before we dive into the specific elements with four valence electrons, let's clarify the concept of valence electrons. Valence electrons are the electrons located in the outermost shell (energy level) of an atom. These electrons are crucial because they determine an atom's chemical behavior and its ability to form chemical bonds with other atoms. Atoms strive for stability, often by achieving a full outer shell of electrons. This usually means eight electrons (the octet rule), although there are exceptions.
Elements with four valence electrons are particularly interesting because they can achieve stability in a few ways: they can gain four electrons, lose four electrons, or share four electrons through covalent bonding. This versatility contributes to the wide range of compounds and materials these elements form.
Group 14: The Carbon Family
Group 14, also known as the carbon family, comprises elements with four valence electrons. This group includes:
- Carbon (C): The cornerstone of organic chemistry and the foundation of life itself.
- Silicon (Si): A vital component in semiconductors and computer chips.
- Germanium (Ge): Used in transistors, fiber optics, and other advanced technologies.
- Tin (Sn): A versatile metal used in alloys, coatings, and soldering.
- Lead (Pb): Historically used in various applications but now increasingly restricted due to its toxicity.
- Flerovium (Fl): A synthetic, highly radioactive element with limited known properties.
Carbon: The Element of Life
Carbon, arguably the most important element in Group 14, forms the backbone of all known life forms. Its unique ability to form strong covalent bonds with other carbon atoms, as well as with hydrogen, oxygen, nitrogen, and sulfur, allows for the creation of a vast array of complex organic molecules. From simple hydrocarbons like methane (CH₄) to the intricate macromolecules like proteins and DNA, carbon's versatility is unmatched.
Carbon's Allotropes: A Tale of Diverse Forms
Carbon exhibits allotropy, meaning it can exist in different structural forms with vastly different properties. These include:
- Diamond: Known for its exceptional hardness, high refractive index, and thermal conductivity. The strong covalent bonds between carbon atoms in a tetrahedral structure create a rigid and incredibly strong material.
- Graphite: A soft, slippery material used in pencils and as a lubricant. Graphite's structure consists of layers of carbon atoms bonded in hexagonal lattices, allowing these layers to slide easily over each other.
- Fullerenes: These cage-like molecules, including buckminsterfullerene (C₆₀), possess unique properties and find applications in nanotechnology and materials science.
- Graphene: A single layer of carbon atoms arranged in a honeycomb lattice. It is incredibly strong, light, and exhibits exceptional electrical conductivity, making it a promising material for various applications, including electronics and composites.
Silicon: The Heart of the Digital Revolution
Silicon, the second element in Group 14, plays a pivotal role in modern technology. Its ability to form semiconductors is crucial for the operation of transistors, integrated circuits, and microprocessors – the building blocks of computers and countless other electronic devices. Silicon's semiconducting properties are due to its ability to control the flow of electrons under specific conditions.
Silicon's Importance in Semiconductors
The unique electronic structure of silicon allows it to be easily doped with other elements (such as boron or phosphorus) to alter its electrical conductivity. This doping process is essential for creating p-type and n-type semiconductors, the foundation of modern electronics. The combination of p-type and n-type silicon forms the basis of transistors and integrated circuits, enabling the miniaturization and remarkable power of modern computing.
Germanium, Tin, and Lead: Diverse Applications
While not as ubiquitous as carbon and silicon, germanium, tin, and lead also find significant applications.
Germanium, known for its high refractive index, is used in fiber optics to transmit light signals efficiently. It also finds applications in transistors and some types of solar cells.
Tin, a relatively soft and malleable metal, is used extensively in alloys to improve their properties. Solder, a crucial material in electronics, often contains tin. Tin coatings are also used to protect steel from corrosion.
Lead, although highly toxic and its use is being phased out in many applications, historically had significant uses in batteries, pipes, and other materials due to its ability to resist corrosion. However, due to its toxicity, its applications are increasingly limited.
Flerovium: A Synthetic Element
Flerovium is a synthetic, radioactive element that has only been produced in small quantities in laboratories. Its properties are not well-understood due to its short half-life and limited availability. Research into flerovium is ongoing, pushing the boundaries of our understanding of the periodic table and the properties of heavy elements.
The Chemistry of Group 14 Elements: Bonding and Reactivity
The chemistry of Group 14 elements is largely dictated by their four valence electrons. Carbon, due to its small size, predominantly forms covalent bonds. Silicon, germanium, tin, and lead also form covalent bonds, but their larger size and lower electronegativity allow them to form some ionic compounds as well.
Covalent Bonding in Group 14
The most prevalent type of bonding in Group 14 compounds is covalent bonding, where atoms share electrons to achieve a stable electron configuration. This leads to the formation of a wide range of molecules and polymeric structures, with properties varying significantly depending on the bonding arrangements and the other atoms involved.
Reactivity Trends
The reactivity of Group 14 elements tends to decrease as you move down the group. Carbon is relatively unreactive at room temperature, requiring high temperatures or specific catalysts to participate in many chemical reactions. Silicon is more reactive than carbon, while germanium, tin, and lead exhibit increasing reactivity.
Environmental Impact and Toxicity
The environmental impact and toxicity of Group 14 elements vary considerably. Carbon, in its various forms, is essential for life and relatively benign in most forms. However, certain carbon-based pollutants, such as greenhouse gases (CO2, CH4), are major contributors to climate change.
Silicon is generally non-toxic, but some silicon compounds can be harmful.
Germanium is relatively low in toxicity.
Tin is also relatively non-toxic in its metallic form, but some tin compounds can be harmful.
Lead is well-known for its significant toxicity. Lead poisoning can lead to severe neurological damage and other health problems. Its use is increasingly restricted due to its hazardous effects.
Conclusion: A Diverse and Important Group
Group 14 elements, with their characteristic four valence electrons, exhibit a remarkable diversity in properties and applications. From the life-sustaining carbon to the technologically vital silicon and the historically important tin and lead, these elements have profoundly shaped human civilization. Understanding their unique characteristics and chemical behavior is crucial in various fields, from materials science and electronics to environmental science and medicine. Ongoing research continues to unveil new insights and applications for these fascinating elements, ensuring their continued importance in the future. Further research is needed, especially in the area of environmentally friendly alternatives to the more toxic elements within this group, promoting sustainable practices and mitigating environmental risks.
Latest Posts
Latest Posts
-
What Is 3 6 In A Fraction
Apr 01, 2025
-
What Are Convection Currents And What Causes Them
Apr 01, 2025
-
Circumference Of A Circle With A Diameter Of 10
Apr 01, 2025
-
What Transition Metals Have A Fixed Charge
Apr 01, 2025
-
What Are Biotic Factors And Abiotic Factors
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Element Has 4 Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
