What Do Elements In Same Period Have In Common
listenit
Mar 27, 2025 · 6 min read
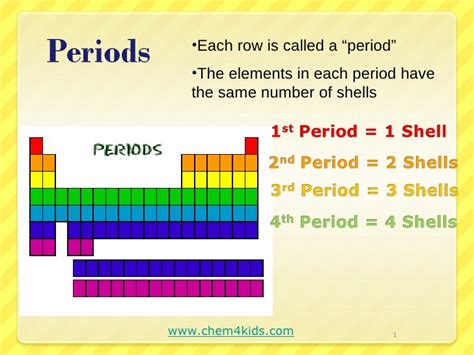
Table of Contents
What Do Elements in the Same Period Have in Common? Exploring Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the relationships between elements is crucial to comprehending chemical reactions and predicting the behavior of matter. One key relationship lies in the elements sharing the same period. But what exactly do elements in the same period have in common? This article delves deep into this question, exploring the underlying principles and examining the fascinating trends that emerge across a period.
The Significance of Periods in the Periodic Table
The periodic table is arranged in a grid format with rows called periods and columns called groups. Periods are horizontal rows, and each period represents a principal energy level or electron shell being filled. This is the fundamental similarity that binds elements within the same period: they all have the same number of electron shells.
The Number of Electron Shells: A Defining Feature
The number of electron shells directly impacts an element's properties. For example, elements in Period 1, hydrogen (H) and helium (He), have only one electron shell. Elements in Period 2, such as lithium (Li) and neon (Ne), have two electron shells, and so on. This shared number of shells influences several key characteristics, as we will explore in detail.
Key Properties Shared by Elements in the Same Period
Elements within the same period exhibit a range of similar properties and predictable trends as we move across the period from left to right. These trends are crucial in understanding chemical reactivity and predicting the behavior of elements.
1. Atomic Radius: A Gradual Decrease
Atomic radius refers to the distance from the nucleus to the outermost electron shell. As we progress across a period from left to right, the atomic radius generally decreases. This is because the number of protons in the nucleus increases, resulting in a stronger positive charge that pulls the electrons closer to the nucleus. While the number of electrons also increases, the added electrons go into the same energy level, not shielding the effect of the increased nuclear charge. Consequently, the outermost electrons are held more tightly, leading to a smaller atomic radius.
2. Ionization Energy: A General Increase
Ionization energy is the energy required to remove an electron from a gaseous atom. As we move across a period, ionization energy generally increases. This is a direct consequence of the decreasing atomic radius. Since the electrons are held more tightly by the increasing nuclear charge, more energy is needed to remove an electron. This trend is crucial in determining the reactivity of elements and their tendency to form ions.
3. Electronegativity: A Steady Rise
Electronegativity measures an atom's ability to attract electrons towards itself in a chemical bond. As we move from left to right across a period, electronegativity generally increases. This is because of the same reason as the ionization energy trend: the increasing nuclear charge pulls the shared electrons more strongly towards the atom. Elements with high electronegativity tend to attract electrons strongly in a chemical bond, often forming covalent bonds with a polar character.
4. Electron Affinity: A Complex Trend
Electron affinity is the energy change that occurs when an atom gains an electron. While there's a general trend of increasing electron affinity across a period, it's less consistent than ionization energy or electronegativity. The trend is complicated by electron shell filling and electron-electron repulsions. For instance, adding an electron to a half-filled or completely filled subshell might be less favorable energetically than adding it to a partially filled subshell.
5. Metallic Character: A Transition from Metal to Nonmetal
The metallic character of elements refers to their tendency to lose electrons and form positive ions. Across a period, metallic character generally decreases. Elements on the left side of the period tend to be metals (easily losing electrons), while elements on the right are nonmetals (more likely to gain electrons). This transition reflects the change in their ionization energy and electronegativity.
6. Chemical Reactivity: A Divergent Pattern
The chemical reactivity of elements varies across a period, reflecting the trends in ionization energy and electronegativity. Alkali metals (Group 1) are highly reactive due to their low ionization energies, readily losing one electron to form +1 ions. Halogens (Group 17) are also highly reactive but gain one electron to form -1 ions due to their high electron affinities. Noble gases (Group 18) are generally unreactive because their electron shells are completely filled. The reactivity pattern is not monotonic across a period but rather shows peaks at the alkali metals and halogens, and a minimum at the noble gases.
Exceptions and Irregularities: Refining the Trends
While the trends discussed above are general, there are exceptions and irregularities. These deviations often arise from the complexities of electron configurations and electron-electron interactions. For example:
- Transition metals: The trends in atomic radius, ionization energy, and electronegativity are less pronounced in transition metals due to the filling of inner d orbitals.
- Anomalous electron configurations: Some elements deviate from the expected electron configuration, leading to altered properties. For example, chromium (Cr) and copper (Cu) have anomalous electron configurations that influence their properties.
- Shielding effects: The inner electrons can shield the outer electrons from the full nuclear charge, partially counteracting the expected trends.
Applications and Implications
Understanding the periodic trends within a period has several crucial applications:
- Predicting chemical reactions: Knowing the relative electronegativity and ionization energies of elements helps predict the type of chemical bonds they will form and the reactivity of the resulting compounds.
- Designing new materials: The properties of elements can be tuned by selecting elements from specific periods with desired characteristics, leading to the development of new materials with tailored properties.
- Understanding biological systems: The properties of elements within periods are essential in understanding the role of elements in biological systems, such as the importance of iron in hemoglobin.
- Developing new technologies: The trends in properties can guide the development of new technologies and materials, such as semiconductors and catalysts.
Conclusion: The Power of Periodicity
Elements within the same period share the fundamental characteristic of possessing the same number of electron shells. This shared feature gives rise to observable trends in atomic radius, ionization energy, electronegativity, metallic character, and chemical reactivity. While some exceptions and irregularities exist, understanding these periodic trends is vital for predicting chemical behavior, designing new materials, and deepening our comprehension of the natural world. The periodic table, with its inherent organization, is a powerful tool that allows us to grasp the intricate relationships and predictable patterns exhibited by elements, ultimately fostering a richer understanding of chemistry. Further exploration of these trends, including the exceptions and nuances, reveals the remarkable depth and complexity of the periodic system. By continually investigating these relationships, we can unlock the potential for new discoveries and technological advancements.
Latest Posts
Latest Posts
-
Unit Weight Of Water In Lb Ft3
Mar 30, 2025
-
What Are The Common Factors Of 4 And 6
Mar 30, 2025
-
What Is The Gcf Of 64 And 32
Mar 30, 2025
-
Write The Condensed Structure For The Molecule Shown
Mar 30, 2025
-
How To Get From Vertex Form To Factored Form
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Do Elements In Same Period Have In Common . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
