Write The Condensed Structure For The Molecule Shown
listenit
Mar 30, 2025 · 5 min read
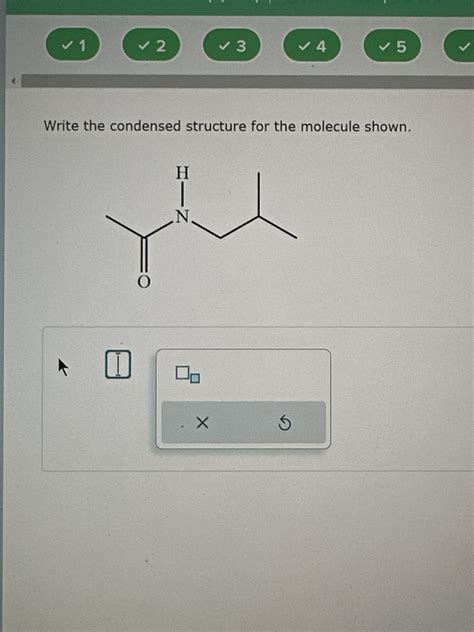
Table of Contents
Understanding and Drawing Condensed Structural Formulas: A Comprehensive Guide
Condensed structural formulas are a simplified way to represent the structure of molecules, offering a balance between detail and brevity. Unlike fully drawn structural formulas that show every bond, condensed formulas prioritize showing the connectivity of atoms while streamlining the representation. Mastering this skill is crucial for anyone studying organic chemistry or related fields. This comprehensive guide will walk you through the process of understanding and drawing condensed structural formulas, covering various complexities and examples.
What is a Condensed Structural Formula?
A condensed structural formula represents the atoms and their connectivity in a molecule in a more compact form than a fully expanded structural formula. It omits the explicit drawing of every single bond, implying bonds between adjacent atoms in a linear sequence. This makes it particularly useful for larger and more complex molecules where drawing every bond would be cumbersome and visually confusing. The key is to understand that the connectivity is still explicitly implied.
Key Elements of Condensed Structural Formulas
Before diving into examples, let's highlight the core elements:
- Parent Chain/Backbone: The longest continuous carbon chain in the molecule forms the basis of the condensed formula.
- Substituents: Atoms or groups of atoms attached to the parent chain are represented as branches or extensions.
- Functional Groups: Important functional groups (like -OH, -COOH, -CHO) are typically retained explicitly to highlight their presence.
- Implicit Bonds: Bonds between atoms are implied by their adjacency within the formula. If two atoms are written next to each other, a bond exists between them.
- Parentheses: Parentheses are used to indicate branching or multiple substituents attached to the same carbon.
How to Draw Condensed Structural Formulas: A Step-by-Step Guide
Let's illustrate with an example. Consider the molecule with the fully drawn structure:
(Insert image of a simple branched alkane, e.g., 2-methylbutane)
Step 1: Identify the Parent Chain: In this case, the longest continuous carbon chain has four carbons, forming a butane backbone.
Step 2: Number the Parent Chain: This is optional but helpful, particularly for more complex molecules. Numbering helps to correctly position substituents.
Step 3: Identify Substituents: We have a methyl group (-CH3) attached to the second carbon atom.
Step 4: Write the Condensed Formula: Starting with the parent chain, we represent it as CH3CHCH2CH3. Then, we place the substituent, indicated by the methyl group, on the appropriate carbon: CH3CH(CH3)CH2CH3. Notice that the parentheses clearly indicate the methyl group is attached to the second carbon.
Working with Different Functional Groups
The principles remain the same, even when dealing with molecules containing different functional groups.
Alcohols (-OH)
Consider the molecule propan-2-ol:
(Insert image of propan-2-ol)
The condensed formula is CH3CH(OH)CH3. Notice how the -OH group, characterizing the alcohol, is explicitly shown, and its position on the second carbon atom is clearly indicated within the parentheses.
Carboxylic Acids (-COOH)
Let's look at ethanoic acid (acetic acid):
(Insert image of ethanoic acid)
Its condensed formula is CH3COOH. The carboxylic acid group (-COOH) is represented directly as this group.
Aldehydes (-CHO)
Consider methanal (formaldehyde):
(Insert image of methanal)
The condensed formula is simply HCHO.
Ketones (C=O)
Consider propanone (acetone):
(Insert image of propanone)
The condensed formula is CH3COCH3. The carbonyl group (=O) is explicitly shown, positioned between two methyl groups.
Dealing with More Complex Structures
As molecules become more complex, the principles remain the same, but careful attention to detail is crucial:
(Insert image of a more complex molecule, e.g., a molecule with multiple branches or rings)
To represent this complex molecule, the strategy is the same: identify the longest carbon chain, number it, and then systematically add substituents with parentheses to indicate their position and branching. This could involve nested parentheses, but always remember to keep track of which branches attach to which carbons.
For cyclic molecules (containing rings), indicate the ring structure explicitly, then add substituents to the ring carbons. For example, methylcyclohexane would be represented as CH3-C6H11, indicating a methyl group (-CH3) attached to a cyclohexane ring (C6H11).
The Importance of Practice
Understanding condensed structural formulas takes practice. Start with simple examples and gradually work your way up to more complex molecules. Plenty of practice problems are available in organic chemistry textbooks and online resources. The more you practice, the more intuitive it will become to translate between fully drawn structures and their condensed counterparts.
Benefits of Using Condensed Structural Formulas
Using condensed structural formulas offers several advantages:
- Space Saving: They are significantly more compact than fully drawn structures, especially for large molecules.
- Clarity: While compact, they still convey the essential information about the connectivity of atoms.
- Ease of Use: They are easier and faster to write compared to fully drawn structures.
- Standardized Representation: They represent a widely accepted and understood form of representing molecular structures.
Common Mistakes to Avoid
- Incorrect placement of substituents: Double-check the numbering of the parent chain and the position of each substituent.
- Missing or extra parentheses: Ensure proper use of parentheses to avoid ambiguity.
- Incorrect representation of functional groups: Pay close attention to the specific representation of functional groups like –OH, -COOH, -CHO, etc.
- Oversimplification: Although condensed formulas are compact, they shouldn’t lose the important information on connectivity.
Conclusion
Mastering the skill of writing and interpreting condensed structural formulas is essential for success in organic chemistry and related disciplines. By understanding the underlying principles and practicing regularly, you can confidently navigate the world of molecular representation and efficiently communicate the structures of complex organic molecules. Remember to practice regularly, focusing on the systematic approach of identifying the parent chain, substituents, and functional groups and utilizing parentheses correctly to represent branching. This will significantly improve your understanding and enhance your ability to work with organic molecules. Remember, this is a fundamental skill that will continue to be relevant throughout your studies and work in science.
Latest Posts
Latest Posts
-
Whats Half Of 1 And 1 2
Apr 01, 2025
-
Why Is Water Liquid At Room Temperature
Apr 01, 2025
-
How To Determine The Density Of A Solid
Apr 01, 2025
-
How Long Does It Take To Drive 1500 Miles
Apr 01, 2025
-
What Is 1 6 As A Percent
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Write The Condensed Structure For The Molecule Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
