What Color Has The Highest Energy
listenit
Mar 15, 2025 · 4 min read
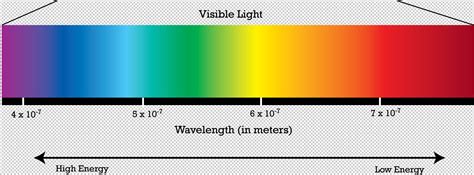
Table of Contents
What Color Has the Highest Energy? Exploring the Physics of Light and Color
The question of which color possesses the highest energy might seem deceptively simple. After all, we associate certain colors with vibrant, energetic feelings. However, the true answer delves into the fascinating world of physics, specifically the properties of light and its electromagnetic spectrum. This exploration will uncover not only the scientific answer but also the nuances involved in interpreting the relationship between color, energy, and our perception.
Understanding the Electromagnetic Spectrum
Before diving into the specifics of color and energy, it's crucial to understand the broader context of the electromagnetic spectrum. This spectrum encompasses all types of electromagnetic radiation, ranging from radio waves with long wavelengths and low energy to gamma rays with short wavelengths and incredibly high energy. Visible light, the portion we can see, occupies a tiny sliver of this vast spectrum.
Within the visible light spectrum, we perceive different wavelengths as different colors. Red light has the longest wavelength and lowest frequency, while violet light has the shortest wavelength and highest frequency. This is a fundamental relationship: wavelength and frequency are inversely proportional.
The Crucial Link: Energy and Frequency
The key to understanding which color has the highest energy lies in the relationship between energy (E), frequency (f), and Planck's constant (h). This relationship is expressed by the following equation:
E = hf
Planck's constant (h) is a fundamental physical constant. This equation reveals that energy is directly proportional to frequency. The higher the frequency of light, the higher its energy.
Since violet light has the highest frequency within the visible spectrum, it consequently possesses the highest energy.
Violet: The Highest Energy Color in the Visible Spectrum
Based on the physics of light and the equation E=hf, the unequivocal answer is violet. Its high frequency translates directly to high energy. This is not merely a subjective perception; it's a fundamental principle of physics.
Beyond the Visible Spectrum: Ultraviolet and Beyond
It's important to note that the visible spectrum is just a small part of the electromagnetic spectrum. Beyond violet, we encounter ultraviolet (UV) radiation, which has even shorter wavelengths and higher frequencies, therefore significantly higher energy than violet light. Beyond UV lies X-rays and gamma rays, which possess exponentially higher energy levels.
However, the question specifically asks about visible light, and within that limited range, violet reigns supreme in terms of energy.
The Perception of Color and Energy: A Subjective Experience
While violet possesses the highest energy in the visible spectrum, our perception of "energy" associated with color is often subjective. We might associate bright, saturated colors like red or orange with high energy, while associating calming colors like blue or green with low energy. This is due to a complex interplay of psychological and cultural factors:
- Psychological Associations: Our brains have evolved to link certain colors with specific experiences and emotions. Red, for example, might be associated with danger or excitement, leading to a perception of high energy.
- Cultural Influences: Cultural contexts shape our interpretation of colors. Different cultures may associate different colors with different concepts and emotions.
- Brightness and Saturation: The brightness and saturation of a color can significantly influence our perception of its energy level. A bright, saturated red will appear more energetic than a dull, muted red.
These psychological and cultural factors play a crucial role in how we perceive the "energy" of a color, but they do not alter the fundamental physics governing the actual energy content of light.
Applications of High-Energy Light: Violet and Beyond
The high energy of violet light and beyond has significant applications in various fields:
- UV Sterilization: UV light's high energy is used to kill bacteria and viruses, making it crucial in sterilization processes in hospitals and other settings.
- Phototherapy: UV light is used in phototherapy to treat certain skin conditions.
- Fluorescence Microscopy: UV light is used to excite fluorescent molecules, enabling high-resolution imaging in biological research.
- X-ray Imaging: X-rays, with their even higher energy, are used in medical imaging to visualize bones and internal organs.
- Gamma-ray Astronomy: Gamma rays, the highest-energy form of electromagnetic radiation, provide insights into the most energetic processes in the universe.
These examples highlight the practical importance of high-energy light across various scientific and technological disciplines.
Conclusion: Science Meets Perception
While violet light holds the title of the highest energy color within the visible spectrum based on the physics of light and its frequency, our perception of "energetic" colors is shaped by psychological and cultural factors. It's crucial to differentiate between the objective, measurable energy content of light and our subjective interpretation of its associated energy. Understanding this distinction allows for a richer appreciation of both the scientific principles and the human experience of color. The relationship between color, energy, and our perception remains a fascinating area of ongoing research and exploration. The next time you see a vibrant violet, remember the powerful energy it carries, both scientifically and perceptually.
Latest Posts
Latest Posts
-
1 Is What Percent Of 7
Mar 15, 2025
-
The Symbol For Sample Standard Deviation Is
Mar 15, 2025
-
What Is The Product Of 7 16 4 3 And 1 2
Mar 15, 2025
-
Is 4 A Rational Number Or Irrational
Mar 15, 2025
-
Salad Dressing Homogeneous Heterogeneous Solution Colloid Suspension
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Color Has The Highest Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
