What Are Three Parts Of An Atp Molecule
listenit
Mar 20, 2025 · 5 min read
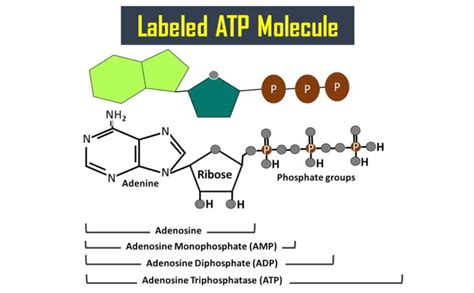
Table of Contents
- What Are Three Parts Of An Atp Molecule
- Table of Contents
- What Are the Three Parts of an ATP Molecule? Understanding Adenosine Triphosphate
- The Tripartite Structure of ATP: Adenine, Ribose, and Phosphate Groups
- 1. Adenine: The Nitrogenous Base
- 2. Ribose: The Five-Carbon Sugar
- 3. Triphosphate Group: The Energy Reservoir
- ATP's Central Role in Cellular Metabolism
- The ATP-ADP Cycle: A Continuous Energy Exchange
- Conclusion: ATP - The Master Molecule of Cellular Energy
- Latest Posts
- Latest Posts
- Related Post
What Are the Three Parts of an ATP Molecule? Understanding Adenosine Triphosphate
Adenosine triphosphate (ATP) is often called the "energy currency" of cells. This vital molecule fuels countless cellular processes, from muscle contraction to protein synthesis and nerve impulse transmission. Understanding its structure is crucial to grasping its function. This article will delve deep into the three components of an ATP molecule, exploring their individual roles and how they collectively contribute to ATP's remarkable energy-storing capabilities.
The Tripartite Structure of ATP: Adenine, Ribose, and Phosphate Groups
The ATP molecule is comprised of three fundamental parts:
- Adenine: A nitrogenous base.
- Ribose: A five-carbon sugar.
- Triphosphate group: A chain of three phosphate groups.
Let's examine each component in detail.
1. Adenine: The Nitrogenous Base
Adenine is a purine base, a type of nitrogen-containing molecule with a double-ring structure. This specific structure is crucial for its interaction with other molecules within the cell. Its ability to form hydrogen bonds with other molecules, particularly uracil (in RNA) and thymine (in DNA), is vital for its role in genetic information storage and transfer. In ATP, adenine is attached to the ribose sugar, forming the nucleoside adenosine.
Key characteristics of adenine in the context of ATP:
- Planar Structure: The relatively flat structure of adenine allows for efficient stacking interactions, which are important in the stability of nucleic acids and ATP itself.
- Hydrogen Bonding: Adenine's ability to form hydrogen bonds is central to its role in base pairing and molecular recognition. This is indirectly important for ATP function, as it contributes to the overall stability of the molecule.
- Hydrophobic Nature: Certain portions of adenine exhibit hydrophobic (water-repelling) properties, impacting how it interacts with the aqueous environment of the cell.
2. Ribose: The Five-Carbon Sugar
Ribose is a pentose sugar, meaning it contains five carbon atoms. It forms the backbone to which the adenine base and the triphosphate group are attached. Ribose exists in a cyclic form in ATP, meaning it forms a ring structure. This ring structure is essential for the molecule's three-dimensional conformation and its interactions with enzymes. The specific configuration of ribose (β-D-ribose) dictates how the other components attach and thus the overall shape of ATP.
Key characteristics of ribose in ATP:
- Cyclic Structure: The cyclic form of ribose provides structural rigidity and stability to the ATP molecule.
- Hydroxyl Groups: The presence of hydroxyl groups (-OH) on the ribose ring contributes to its polarity and its ability to interact with water molecules. This is critical given the primarily aqueous environment inside cells.
- Attachment Points: Specific carbon atoms on the ribose ring provide the attachment points for both the adenine base and the phosphate groups, accurately positioning them for optimal function.
3. Triphosphate Group: The Energy Reservoir
The triphosphate group is where the magic happens. This chain of three phosphate groups (alpha, beta, and gamma) is linked together by high-energy phosphoanhydride bonds. These bonds are high-energy because they store significant potential energy. The energy released when these bonds are broken is what powers numerous cellular processes. It's important to note that the energy is not stored within the bonds themselves, but rather the release of energy is a result of the rearrangement of charges and resonance stabilization. The products of hydrolysis (breaking the bond) are more stable than the reactants, releasing free energy in the process.
Understanding the High-Energy Phosphate Bonds:
The high-energy nature of these bonds is due to several factors:
- Electrostatic Repulsion: The negatively charged phosphate groups repel each other. This repulsion creates inherent instability, making the bonds prone to hydrolysis (breaking down with the addition of water).
- Resonance Stabilization: The products of hydrolysis (ADP and inorganic phosphate) have greater resonance stabilization than ATP. This increased stability releases energy.
- Hydration: The products of hydrolysis are more easily hydrated than ATP, further contributing to their stability and the release of energy.
The Role of Each Phosphate Group:
- Alpha (α) phosphate: This is the phosphate group closest to the ribose sugar.
- Beta (β) phosphate: This is the middle phosphate group.
- Gamma (γ) phosphate: This is the terminal phosphate group, and it is most commonly cleaved during energy transfer.
When ATP is hydrolyzed, typically the bond between the beta and gamma phosphates is broken, releasing a significant amount of energy and producing adenosine diphosphate (ADP) and inorganic phosphate (Pi). This energy is then coupled to other cellular reactions to drive them forward.
ATP's Central Role in Cellular Metabolism
ATP serves as the primary energy source for a wide array of cellular activities, including:
- Muscle Contraction: The sliding filament mechanism in muscle relies heavily on ATP hydrolysis to power the interactions between actin and myosin.
- Active Transport: ATP-powered pumps move ions and molecules across cell membranes against their concentration gradients.
- Protein Synthesis: The synthesis of proteins requires energy from ATP to drive the formation of peptide bonds.
- Nerve Impulse Transmission: The transmission of nerve impulses relies on ATP-dependent ion channels and pumps.
- DNA Replication and Repair: DNA replication and repair processes require ATP to fuel the enzymatic reactions involved.
- Cell Signaling: Many cell signaling pathways utilize ATP to regulate cellular processes.
ATP's ability to readily donate and accept phosphate groups allows it to participate in numerous phosphorylation reactions, modifying the activity of proteins and enzymes. This regulatory role extends beyond simply providing energy; it's a key player in coordinating cellular functions.
The ATP-ADP Cycle: A Continuous Energy Exchange
ATP is not a static molecule; instead, it participates in a dynamic cycle with ADP. The continuous conversion between ATP and ADP ensures a constant supply of energy to meet the cell's demands. Cellular respiration, primarily through oxidative phosphorylation, regenerates ATP from ADP and Pi, ensuring a continuous flow of energy to power various cellular processes. This process involves the electron transport chain and chemiosmosis within the mitochondria.
Conclusion: ATP - The Master Molecule of Cellular Energy
The three-part structure of the ATP molecule—adenine, ribose, and the triphosphate group—is elegantly designed to fulfill its critical role as the energy currency of life. The high-energy phosphate bonds provide readily available energy for a vast range of cellular processes, essential for maintaining life. Understanding the individual components and their interactions is fundamental to comprehending the complexities of cellular metabolism and the dynamic nature of life itself. Further exploration into the intricate mechanisms of ATP synthesis and utilization continues to be a significant area of biological research, revealing the astounding efficiency and elegance of this remarkable molecule.
Latest Posts
Latest Posts
-
What Percent Of 35 Is 70
Mar 21, 2025
-
What Is A 21 Out Of 25
Mar 21, 2025
-
Which Gas Is The Most Abundant In The Atmosphere
Mar 21, 2025
-
What Is Electronic Configuration Of Carbon
Mar 21, 2025
-
Which Electromagnetic Waves Have The Longest Wavelengths
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Are Three Parts Of An Atp Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
