What Are The Three Parts Of Atp Molecule
listenit
Mar 31, 2025 · 6 min read
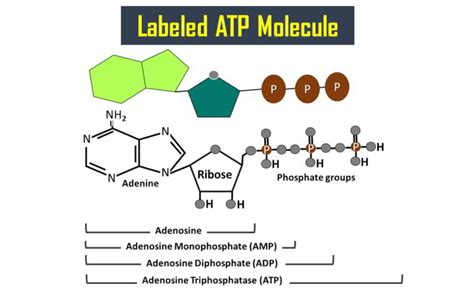
Table of Contents
What are the Three Parts of an ATP Molecule? Unlocking the Energy Currency of Life
Adenosine triphosphate (ATP) is the fundamental energy currency of all living cells. This remarkable molecule fuels countless cellular processes, from muscle contraction and nerve impulse transmission to protein synthesis and DNA replication. Understanding its structure is crucial to understanding how it performs this vital role. This comprehensive guide delves into the three core components of the ATP molecule, exploring their individual roles and how their interaction enables ATP's function as the cell's powerhouse.
The Tripartite Structure: Adenine, Ribose, and Phosphate Groups
The ATP molecule is composed of three distinct parts:
- Adenine: A nitrogenous base.
- Ribose: A five-carbon sugar.
- Triphosphate group: A chain of three phosphate groups.
Let's examine each component in detail:
1. Adenine: The Nitrogenous Base
Adenine is a purine base, a type of nitrogen-containing molecule with a double-ring structure. Its specific arrangement of nitrogen and carbon atoms allows it to form strong hydrogen bonds with its complementary base, thymine (in DNA) or uracil (in RNA). In the context of ATP, adenine's role is primarily structural. It contributes to the overall molecular structure, influencing the molecule's stability and interaction with enzymes. Its specific chemical properties are essential for the recognition and binding of ATP by various enzymes involved in energy transfer. While not directly involved in the energy transfer itself, adenine is an integral part of ATP's identity, ensuring it's recognized by the cellular machinery that utilizes its energy. The presence of adenine in the ATP molecule also allows for highly specific interactions with other molecules, avoiding unintended reactions and energy expenditure.
2. Ribose: The Five-Carbon Sugar
Ribose is a pentose sugar, meaning it contains five carbon atoms. In ATP, it's specifically a β-D-ribose, a particular isomer with a specific spatial arrangement of its atoms. This ribose molecule forms the backbone of the ATP molecule, connecting the adenine base to the triphosphate group. Its hydroxyl (-OH) groups play a crucial role in the molecule's reactivity and interaction with enzymes. The specific arrangement of the ribose molecule influences the three-dimensional structure of ATP, determining its ability to bind to enzymes involved in ATP synthesis and hydrolysis. The position and orientation of the hydroxyl groups are critical for the precise positioning of the phosphate groups, which are essential for energy transfer. The ribose sugar's role extends beyond simple structural support; it actively participates in the chemical processes that allow ATP to function as an energy carrier.
3. Triphosphate Group: The Energy Reservoir
The triphosphate group is the powerhouse of the ATP molecule. It consists of three phosphate groups (P) linked together in a chain: alpha (α), beta (β), and gamma (γ). These phosphate groups carry a significant negative charge, causing electrostatic repulsion between them. This repulsion makes the phosphate bonds relatively unstable and high in energy. This instability is precisely what makes ATP such an effective energy storage and transfer molecule.
The energy stored in ATP resides primarily in the phosphoanhydride bonds between the phosphate groups. These bonds are high-energy bonds because breaking them releases a significant amount of energy that the cell can then harness for various processes. The hydrolysis of ATP – the breaking of a phosphate bond – is a highly exergonic reaction, meaning it releases energy spontaneously. This energy release is coupled with other endergonic (energy-requiring) reactions within the cell, making them energetically favorable.
The process of ATP hydrolysis typically involves the removal of the terminal phosphate group (γ-phosphate), producing adenosine diphosphate (ADP) and inorganic phosphate (Pi). This reaction releases approximately 7.3 kilocalories of energy per mole of ATP under standard conditions. This energy is then used to drive various cellular processes.
The difference between ATP, ADP, and AMP (adenosine monophosphate) lies in the number of phosphate groups they possess. ADP has two phosphate groups and AMP only one. The cyclical process of ATP hydrolysis to ADP and subsequent regeneration of ATP from ADP through cellular respiration maintains the cell's energy balance. The cell constantly replenishes its ATP supply to meet the ongoing demand for energy.
The Role of ATP in Cellular Processes
ATP's central role as an energy carrier stems directly from its unique three-part structure. The high-energy phosphate bonds, facilitated by the triphosphate group's instability, provide the energy needed for numerous cellular functions. Examples include:
- Muscle Contraction: ATP powers the myosin-actin interaction, leading to muscle fiber shortening and movement.
- Active Transport: ATP-driven pumps move ions and molecules across cell membranes against their concentration gradients. This is essential for maintaining cellular homeostasis.
- Nerve Impulse Transmission: The propagation of nerve impulses relies on ATP-dependent ion pumps maintaining the electrochemical gradients across neuronal membranes.
- Protein Synthesis: The synthesis of proteins, a fundamental process for cell growth and function, requires ATP to drive the ribosome's actions and the amino acid's binding.
- DNA Replication and Repair: The accurate replication and repair of DNA, critical for maintaining genetic integrity, rely heavily on ATP-dependent enzymes.
- Cellular Signaling: ATP is involved in various cellular signaling pathways, acting as a signaling molecule and a regulator of enzyme activity.
ATP Synthesis: How ATP is Made
The cell continuously produces ATP to meet the ever-present energy demands. The primary method of ATP synthesis is through cellular respiration, a process that occurs in the mitochondria of eukaryotic cells. Cellular respiration involves a series of reactions that break down glucose and other fuels to release energy. This energy is then harnessed to produce ATP from ADP and Pi. This process is highly efficient, producing a significant number of ATP molecules from a single glucose molecule.
Another significant method of ATP production is oxidative phosphorylation, occurring in the inner mitochondrial membrane. A proton gradient is established across the membrane, and the flow of protons back across the membrane through ATP synthase drives the synthesis of ATP. This process relies on the electron transport chain, which utilizes oxygen as the final electron acceptor.
Photoautotrophs, like plants and algae, also use photophosphorylation to produce ATP, using light energy to generate a proton gradient across the thylakoid membranes in chloroplasts.
The Importance of Understanding ATP's Structure
A deep understanding of the three components of the ATP molecule – adenine, ribose, and the triphosphate group – is critical for comprehending its function as the central energy carrier in all living cells. The unique chemical properties of each component, and their specific arrangement, contribute to the molecule's overall stability and its capacity for efficient energy storage and release. The high-energy phosphate bonds, the result of electrostatic repulsion within the triphosphate group, are central to ATP's ability to power countless biological processes.
Further research into the intricate mechanisms governing ATP synthesis and hydrolysis continues to uncover new facets of its crucial role in cellular life. This includes investigations into the regulation of ATP production, the roles of specific enzymes in ATP metabolism, and the development of novel therapeutic strategies targeting ATP-dependent processes. By unraveling the complexities of ATP's structure and function, we gain a deeper appreciation for the fundamental processes that sustain life itself. The ongoing research in this area continues to reveal the intricacies of this essential molecule, enriching our understanding of biology and paving the way for potential advancements in medicine and biotechnology. The three parts of the ATP molecule, while seemingly simple, represent a masterpiece of biological engineering, fundamental to the existence and functionality of life on Earth.
Latest Posts
Latest Posts
-
What Is 15 As A Fraction
Apr 02, 2025
-
What Is The Lcm For 6 And 10
Apr 02, 2025
-
Which Organelles Supply Energy To The Cell
Apr 02, 2025
-
Why Is Density A Physical Property
Apr 02, 2025
-
Is Koh A Base Or Acid
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are The Three Parts Of Atp Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
