What Are The Rows On The Periodic Table Called
listenit
Mar 16, 2025 · 6 min read
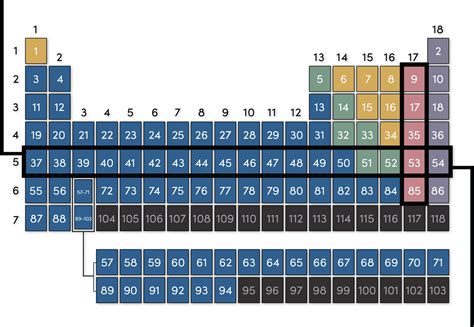
Table of Contents
What are the Rows on the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While most people are familiar with the columns, known as groups or families, the horizontal rows also hold significant meaning and are known as periods. Understanding periods is crucial for comprehending the trends and patterns in elemental properties. This article delves deep into the nature of periods, exploring their significance, the properties they reveal, and their impact on the overall organization of the periodic table.
Understanding Periods: More Than Just a Horizontal Arrangement
The periods on the periodic table represent the principal energy levels, or shells, in which electrons orbit the atom's nucleus. Each period corresponds to a particular value of the principal quantum number (n), a fundamental concept in quantum mechanics describing the energy level of an electron. The first period, for example, contains elements with electrons only in the first energy level (n=1), the second period with electrons in the second energy level (n=2), and so on.
This seemingly simple organization is incredibly powerful. The number of elements in each period isn't arbitrary; it's directly related to the maximum number of electrons that can occupy each energy level. This number is determined by the formula 2n², where 'n' is the principal quantum number. This means:
- Period 1 (n=1): Holds a maximum of 2 electrons (2 x 1² = 2). This period contains only Hydrogen (H) and Helium (He).
- Period 2 (n=2): Holds a maximum of 8 electrons (2 x 2² = 8). This period encompasses elements like Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), and Neon (Ne).
- Period 3 (n=3): Also holds a maximum of 8 electrons (although the 3d subshell isn't filled yet). This period includes Sodium (Na) to Argon (Ar).
- Period 4 (n=4): Holds up to 18 electrons, accommodating the filling of both the 4s and 3d orbitals. This period starts with Potassium (K) and ends with Krypton (Kr).
- Period 5 (n=5): Similar to Period 4, it can accommodate 18 electrons due to the filling of the 5s and 4d orbitals, ranging from Rubidium (Rb) to Xenon (Xe).
- Period 6 (n=6): This period is unique, holding up to 32 electrons due to the inclusion of the 6s, 4f, 5d orbitals. This long period starts with Cesium (Cs) and ends with Radon (Rn). The lanthanides are nestled within this period.
- Period 7 (n=7): The longest period, accommodating up to 32 electrons (though incomplete as of current knowledge), including the 7s, 5f, 6d orbitals, starting with Francium (Fr) and ending with the currently synthesized element Tennessine (Ts). The actinides are placed within this period.
The Significance of Periodicity in Chemical Properties
The arrangement of elements in periods reveals crucial trends in their properties. As we move across a period from left to right, the atomic number increases, meaning the number of protons and electrons increases. This systematic increase has a profound impact on several key properties:
-
Atomic Radius: Generally, atomic radius decreases across a period. This is because the increasing nuclear charge attracts the electrons more strongly, pulling them closer to the nucleus.
-
Ionization Energy: Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. The stronger nuclear attraction makes it harder to remove an electron.
-
Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, also increases across a period. The increased nuclear charge enhances the atom's pull on shared electrons.
-
Metallic Character: Metallic character generally decreases across a period. As we move right, elements tend to gain electrons, becoming more non-metallic in their behavior.
Beyond the Basic Trends: A Deeper Look into Periodicity
While the general trends described above provide a good starting point, the reality is more nuanced. The d-block elements (transition metals) and the f-block elements (lanthanides and actinides) introduce complexities to the periodic trends. These elements exhibit less regular variations in their properties due to the subtle differences in electron configurations and shielding effects.
For instance, the transition metals show relatively small variations in atomic radii across a period due to the gradual filling of the inner d orbitals. These inner electrons provide some shielding from the nuclear charge, mitigating the expected decrease in atomic radius. Similarly, the lanthanides and actinides exhibit very similar chemical properties due to the filling of the f orbitals, which are less involved in chemical bonding.
The Role of Electron Configuration in Periodicity
The underlying reason for the observed periodic trends lies in the electron configuration of the elements. Electrons occupy specific energy levels and sublevels (s, p, d, f), and the filling of these sublevels follows specific rules (Aufbau principle, Hund's rule, Pauli exclusion principle). The outermost electrons, also known as valence electrons, are particularly important in determining an element's chemical behavior and reactivity.
Elements in the same period have their valence electrons in the same principal energy level. This similarity in electron configuration leads to similar chemical properties, despite differences in the number of protons and inner electrons. However, as we move across a period, the number of valence electrons increases, leading to changes in bonding behavior and chemical reactivity.
Predicting Properties Based on Period and Group: A Powerful Tool
Understanding the periods allows chemists to predict the properties of elements based on their position in the periodic table. For instance, knowing an element is in Period 3 and Group 17 (halogens) allows us to anticipate its high electronegativity, strong oxidizing power, and tendency to form -1 ions. This predictive power is fundamental in chemical research and applications.
The Periodic Table: A Dynamic and Evolving System
The periodic table is not a static entity; it continues to evolve as our understanding of atomic structure and new elements improves. The discovery of new synthetic elements, primarily in the actinide and transactinide regions, necessitates updates to the table. These additions further highlight the significance of periods as a framework for organizing our understanding of matter's fundamental building blocks.
Applications of Understanding Periods
The understanding of periods and their significance extends far beyond the realm of theoretical chemistry. It's deeply intertwined with various practical applications:
- Material Science: Predicting and designing materials with specific properties, such as conductivity, strength, or reactivity, heavily relies on understanding periodic trends.
- Drug Discovery: Understanding the properties of elements within periods helps in designing drugs with specific interactions with biological systems.
- Catalysis: The catalytic activity of elements often depends on their position within the periodic table, and an understanding of periods is vital in designing efficient catalysts.
- Nuclear Chemistry: The understanding of periods is crucial in studying radioactive decay and nuclear reactions.
Conclusion: The Unfolding Story of Periods
The rows on the periodic table, the periods, are more than just a horizontal arrangement. They represent a fundamental organizing principle reflecting the underlying quantum mechanical structure of atoms. Understanding periods allows us to predict the properties of elements, understand their behavior, and apply this knowledge to various fields, from material science to medicine. The periodic table, with its periods and groups, remains a powerful tool for navigating the fascinating world of chemistry and its endless possibilities. The continued exploration and understanding of periods will undoubtedly continue to shape advancements in countless scientific and technological domains. The elegant simplicity of this arrangement belies the deep complexity and significance it holds for the entire field of chemistry.
Latest Posts
Latest Posts
-
What Is 1 6 Of 24
Mar 16, 2025
-
B 13 3b 13 8 13
Mar 16, 2025
-
How Many Electrons Can The D Sublevel Hold
Mar 16, 2025
-
The Most Abundant Gas In The Atmosphere Is
Mar 16, 2025
-
Common Multiple Of 5 And 9
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Are The Rows On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
