How Many Electrons Can The D Sublevel Hold
listenit
Mar 16, 2025 · 6 min read
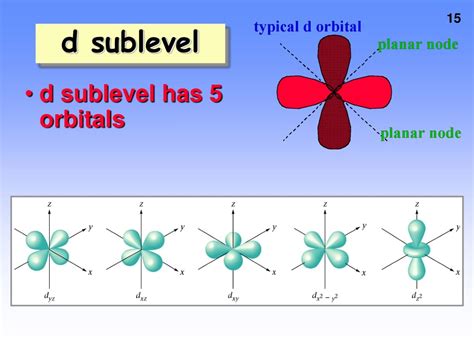
Table of Contents
How Many Electrons Can the d Sublevel Hold? A Deep Dive into Electron Configuration
Understanding electron configuration is fundamental to comprehending the behavior of atoms and molecules. A key aspect of this is grasping the capacity of each electron sublevel. This article delves into the specifics of the d sublevel, explaining not only how many electrons it can hold but also the underlying principles that determine this capacity. We will explore the quantum numbers, orbital shapes, and the implications for chemical bonding and properties.
Understanding Electron Sublevels and Quantum Numbers
Before diving into the d sublevel, let's review the basics of electron configuration. Electrons within an atom occupy specific energy levels, often visualized as shells. Each shell is further divided into sublevels, designated as s, p, d, and f. These sublevels represent different regions of space where electrons are most likely to be found. The number of electrons each sublevel can hold is dictated by quantum numbers.
The Four Quantum Numbers: Defining Electron Properties
Four quantum numbers describe the properties of an electron within an atom:
-
Principal Quantum Number (n): This number designates the electron shell (energy level) and can have any positive integer value (1, 2, 3...). Higher n values indicate higher energy levels and greater distance from the nucleus.
-
Azimuthal Quantum Number (l): This number specifies the sublevel within a shell. It ranges from 0 to n - 1. l = 0 corresponds to the s sublevel, l = 1 to the p sublevel, l = 2 to the d sublevel, and l = 3 to the f sublevel.
-
Magnetic Quantum Number (ml): This number defines the specific orbital within a sublevel. It ranges from -l to +l, including 0. For example, the p sublevel (l = 1) has three orbitals (ml = -1, 0, +1).
-
Spin Quantum Number (ms): This describes the intrinsic angular momentum of an electron, often visualized as spin. It can have only two values: +1/2 (spin up) or -1/2 (spin down). This is the Pauli Exclusion Principle in action – no two electrons in an atom can have the same four quantum numbers.
The d Sublevel: Orbitals and Electron Capacity
The d sublevel is characterized by an azimuthal quantum number (l) of 2. This means it has five orbitals, each capable of holding two electrons (one with spin up and one with spin down). The five ml values for the d sublevel are -2, -1, 0, +1, and +2.
The Shape of d Orbitals: A Visual Representation
Unlike the spherical s orbitals and dumbbell-shaped p orbitals, d orbitals exhibit more complex shapes. Four of the d orbitals have a cloverleaf shape with four lobes, while one has a unique shape with two lobes along the z-axis and a donut-shaped region in the xy-plane. These complex shapes are crucial for understanding the directional nature of chemical bonds involving d electrons.
Filling the d Sublevel: The Aufbau Principle and Hund's Rule
The filling of electron sublevels follows specific rules:
-
Aufbau Principle: Electrons fill the lowest energy levels first. Generally, the order is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on. However, exceptions exist due to electron-electron interactions.
-
Hund's Rule: Within a subshell, electrons will individually occupy each orbital before doubling up in any one orbital. This maximizes the total spin and minimizes electron-electron repulsion.
Because the d sublevel has five orbitals, it can hold a maximum of 10 electrons (5 orbitals × 2 electrons/orbital). This is crucial for understanding the properties of transition metals, which are characterized by partially filled d sublevels.
Transition Metals and the Importance of the d Sublevel
Transition metals, located in the middle of the periodic table, are defined by their partially filled d orbitals. The presence of these d electrons significantly influences their properties:
-
Variable Oxidation States: Transition metals can exhibit multiple oxidation states due to the variable number of d electrons that can participate in chemical bonding. This leads to a wide range of chemical compounds with diverse properties.
-
Colored Compounds: Many transition metal compounds are brightly colored. This is because d electrons can absorb visible light, causing electronic transitions between different d orbitals. The specific color depends on the metal ion and its ligands (the molecules or ions surrounding it).
-
Catalytic Activity: Transition metals are often excellent catalysts. Their partially filled d orbitals allow them to readily accept or donate electrons, facilitating chemical reactions. This is crucial in many industrial processes and biological systems.
-
Magnetic Properties: The presence of unpaired d electrons results in paramagnetism (attraction to magnetic fields). Some transition metal compounds exhibit ferromagnetism (strong attraction to magnetic fields), making them useful in magnets and other applications.
Exceptions and Irregularities in Electron Configuration
While the Aufbau principle provides a general guideline for electron configuration, exceptions do occur. For example, chromium (Cr) and copper (Cu) have unusual configurations. Chromium's configuration is [Ar] 3d⁵ 4s¹, not the expected [Ar] 3d⁴ 4s². Similarly, copper has a configuration of [Ar] 3d¹⁰ 4s¹, rather than [Ar] 3d⁹ 4s². These exceptions arise from the stability gained by having half-filled or completely filled d subshells. The energy difference between the 3d and 4s sublevels is relatively small, making these exceptions energetically favorable.
The d Sublevel and Chemical Bonding
The d electrons play a critical role in chemical bonding, especially in coordination complexes (complex ions). In these complexes, the transition metal ion is surrounded by ligands, which donate electron pairs to the metal. The bonding involves interactions between the d orbitals of the metal and the orbitals of the ligands. The specific geometry and properties of the complex are determined by the nature of the ligands and the number of d electrons on the metal ion. This is a complex area of chemistry, involving concepts like crystal field theory and ligand field theory, which explain the electronic structure and spectral properties of coordination complexes.
Conclusion: A Fundamental Aspect of Atomic Structure
The d sublevel, with its capacity to hold 10 electrons, is a fundamental component of atomic structure. Its unique properties, influenced by the shapes of its orbitals and the rules governing electron filling, have profound consequences for the chemical behavior of atoms, particularly transition metals. Understanding the d sublevel is key to comprehending the properties of countless compounds and materials, ranging from colorful pigments to vital biological catalysts. Its influence extends far beyond simple electron counting, impacting diverse areas of chemistry, physics, and materials science. Further exploration of crystal field theory, ligand field theory, and molecular orbital theory will provide a more complete understanding of the complex roles played by these crucial d electrons. The importance of understanding the nuances of electron configuration in the d sublevel cannot be overstated. It forms the basis of our comprehension of many crucial aspects of the chemical and physical world around us.
Latest Posts
Latest Posts
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The D Sublevel Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
