What Are The Columns Of The Periodic Table Called
listenit
Mar 17, 2025 · 6 min read
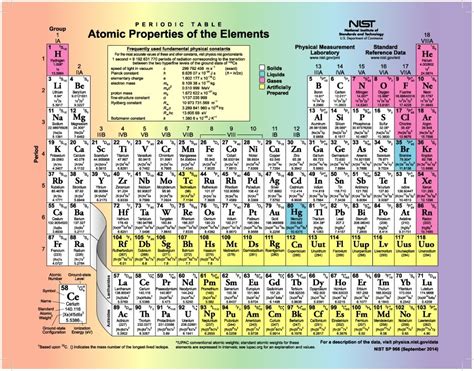
Table of Contents
What Are the Columns of the Periodic Table Called? A Deep Dive into Groups and Families
The periodic table, that iconic chart adorning countless science classrooms, is more than just a neatly organized list of elements. It's a powerful tool reflecting fundamental chemical properties and relationships. While the rows, known as periods, represent increasing electron shells, the columns, or vertical groups, are equally crucial. Understanding what these columns are called and what they signify is fundamental to grasping the intricacies of chemistry. This article delves into the naming conventions, the underlying principles, and the significance of the periodic table's columns, often referred to as groups or families.
The Nomenclature: Groups vs. Families
The terms "groups" and "families" are often used interchangeably to describe the columns of the periodic table. Both terms highlight the shared properties among elements within the same vertical column. However, subtle differences in usage exist.
-
Groups: This is the more formal and widely accepted term used in scientific literature and educational settings. The groups are numbered from 1 to 18, a system adopted by the International Union of Pure and Applied Chemistry (IUPAC) in 1985. This standardized numbering system replaced the older, more confusing A and B group designations.
-
Families: This term is often preferred in introductory chemistry courses, conveying the concept of elements sharing similar characteristics and "belonging" together like members of a family. While less formal, it's a useful mnemonic to help students remember the groupings.
The 18 Groups: A Detailed Look
Let's explore each of the 18 groups, highlighting their defining characteristics and notable members:
Group 1: Alkali Metals
This group is characterized by its highly reactive elements. They readily lose one electron to form +1 ions, exhibiting low electronegativity and ionization energy. They are soft, silvery-white metals with low densities.
- Key Members: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr)
- Key Properties: Highly reactive, low ionization energy, low electronegativity, soft metals, low density.
Group 2: Alkaline Earth Metals
Similar to alkali metals, alkaline earth metals are also reactive, though less so than their Group 1 counterparts. They readily lose two electrons to form +2 ions. They are harder, denser, and have higher melting points than alkali metals.
- Key Members: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra)
- Key Properties: Reactive (less so than alkali metals), form +2 ions, harder than alkali metals, higher density and melting points.
Groups 3-12: Transition Metals
This block comprises the transition metals, known for their variable oxidation states and the formation of colored compounds. Their electronic configuration involves the filling of d orbitals, leading to a variety of chemical behaviors.
- Key Members: Iron (Fe), Copper (Cu), Zinc (Zn), Gold (Au), Platinum (Pt) – Numerous other elements fall within this block.
- Key Properties: Variable oxidation states, formation of colored compounds, good conductors of electricity and heat, high melting points, often act as catalysts.
Group 13: Boron Group
This group shows a gradual transition from metalloid (Boron) to metal character as you move down the group. They generally have three valence electrons.
- Key Members: Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl)
- Key Properties: Variable properties; Boron is a metalloid, while the rest are metals.
Group 14: Carbon Group
This group demonstrates significant diversity, ranging from non-metal (Carbon) to metalloids (Silicon, Germanium) and metals (Tin, Lead). They have four valence electrons, capable of forming a variety of bonds.
- Key Members: Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb)
- Key Properties: Variety of properties reflecting the transition from nonmetal to metal; crucial role of Carbon in organic chemistry.
Group 15: Pnictogens
This group includes elements displaying varied properties, from nonmetal (Nitrogen, Phosphorus) to metalloids (Arsenic, Antimony) and metal (Bismuth). They have five valence electrons, often forming covalent compounds.
- Key Members: Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), Bismuth (Bi)
- Key Properties: Variety of properties (nonmetals, metalloids, metals); crucial roles in biological processes (Nitrogen, Phosphorus).
Group 16: Chalcogens
This group shows a progression from nonmetal (Oxygen, Sulfur) to metalloids (Selenium, Tellurium) and metal (Polonium). They have six valence electrons and frequently form anions with a -2 charge.
- Key Members: Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), Polonium (Po)
- Key Properties: Important in biological processes (Oxygen, Sulfur); variety of properties from nonmetal to metal.
Group 17: Halogens
The halogens are highly reactive nonmetals, characterized by their tendency to gain one electron to achieve a stable electron configuration. They form -1 ions (halide ions).
- Key Members: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At)
- Key Properties: Highly reactive, form -1 ions (halide ions), diatomic molecules.
Group 18: Noble Gases
These elements are extremely unreactive due to their full valence electron shells, making them chemically inert. They are monatomic gases under normal conditions.
- Key Members: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn)
- Key Properties: Inert, full valence electron shells, monatomic gases.
Beyond the Groups: Understanding Trends and Relationships
The arrangement of elements into groups isn't arbitrary. The shared properties within each group reflect underlying periodic trends:
- Atomic Radius: Generally increases down a group due to the addition of electron shells.
- Ionization Energy: Generally decreases down a group due to increased shielding effect and atomic size.
- Electronegativity: Generally decreases down a group due to increased atomic size and shielding.
- Electron Affinity: Shows a less clear-cut trend down a group but often decreases.
These trends are critical for predicting chemical behavior and understanding reactivity. The periodic table’s arrangement enables us to extrapolate properties and understand reactivity patterns without needing to perform extensive experiments on each individual element.
The Significance of Group Classification
The classification of elements into groups is paramount for several reasons:
-
Predictive Power: Knowing an element's group allows us to predict its likely chemical behavior, reactivity, and properties. This predictive power is invaluable in various fields like material science, drug design, and industrial chemistry.
-
Understanding Chemical Reactions: Grouping allows us to grasp the underlying principles driving chemical reactions. Elements within the same group react similarly, offering valuable insight into reaction mechanisms and product formation.
-
Simplifying Complex Systems: The periodic table organizes the vast number of elements into manageable groups, simplifying the study of chemical systems. It's a tool for organizing complex information and making it easier to understand.
-
Educational Tool: The periodic table is an essential educational tool, helping students understand chemical principles and relationships in a visually accessible manner.
Conclusion: The Periodic Table - More Than Just a Chart
The columns of the periodic table, known as groups or families, are far more than mere vertical alignments. They are fundamental to understanding the organization and behavior of the elements. Each group displays distinct characteristics reflecting periodic trends and underlying electronic configurations. By understanding the groups and the trends associated with them, we gain invaluable insights into the world of chemistry, enabling prediction, simplification, and a deeper appreciation of the natural world. The periodic table is not merely a chart; it’s a powerful tool that unlocks the secrets of chemical behavior and opens up exciting avenues of scientific exploration.
Latest Posts
Latest Posts
-
What Is The Molar Mass Of Sodium Chloride
Mar 17, 2025
-
What Is The Least Common Multiple For 5 And 6
Mar 17, 2025
-
Least Common Multiple 9 And 7
Mar 17, 2025
-
What Is The Square Root For 196
Mar 17, 2025
-
What Is 35 In Fraction Form
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Are The Columns Of The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
