What Is The Molar Mass Of Sodium Chloride
listenit
Mar 17, 2025 · 6 min read
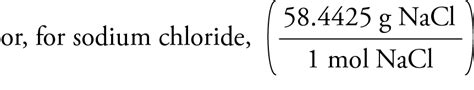
Table of Contents
What is the Molar Mass of Sodium Chloride? A Deep Dive into Atomic Weights and Chemical Calculations
Sodium chloride, commonly known as table salt, is a ubiquitous compound found in countless applications, from seasoning food to industrial processes. Understanding its properties, particularly its molar mass, is crucial in various scientific fields, including chemistry, biochemistry, and materials science. This article delves into the concept of molar mass, explains how to calculate the molar mass of sodium chloride, and explores its significance in different contexts.
Understanding Molar Mass
Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry representing Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of particles, whether they are atoms, molecules, ions, or formula units. Essentially, it's a convenient way to relate the microscopic world of atoms and molecules to the macroscopic world of grams and kilograms that we experience directly. The molar mass is numerically equal to the atomic weight (or molecular weight) of the substance, but with the unit grams per mole (g/mol).
Atomic Weight vs. Molar Mass: A Subtle Difference
While often used interchangeably, there's a subtle distinction. Atomic weight refers to the average mass of an atom of an element, considering the relative abundance of its isotopes. This is a dimensionless quantity. Molar mass, on the other hand, is the mass of one mole of a substance and has the unit g/mol. The numerical values are the same, but the units distinguish them.
Calculating the Molar Mass of Sodium Chloride (NaCl)
Sodium chloride (NaCl) is an ionic compound formed by the electrostatic attraction between sodium (Na<sup>+</sup>) and chloride (Cl<sup>-</sup>) ions. To calculate its molar mass, we need the atomic weights of sodium and chlorine.
Atomic Weights of Sodium and Chlorine
- Sodium (Na): The atomic weight of sodium is approximately 22.99 g/mol. This value is an average reflecting the natural abundance of sodium isotopes.
- Chlorine (Cl): The atomic weight of chlorine is approximately 35.45 g/mol. Similar to sodium, this is an average reflecting the isotopic composition of chlorine found in nature.
Calculating the Molar Mass of NaCl
The molar mass of NaCl is simply the sum of the atomic weights of its constituent elements:
Molar mass of NaCl = Atomic weight of Na + Atomic weight of Cl
Molar mass of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
Therefore, the molar mass of sodium chloride is approximately 58.44 g/mol. This means that one mole of NaCl weighs 58.44 grams.
Significance of Molar Mass in Various Applications
The molar mass of sodium chloride is a fundamental piece of information with wide-ranging applications in various scientific and industrial settings.
1. Stoichiometric Calculations
Molar mass is crucial for stoichiometric calculations, which involve determining the quantities of reactants and products in chemical reactions. Using the molar mass, we can convert between mass and moles, allowing us to accurately predict the amounts of substances involved in a reaction. For example, in a reaction involving NaCl, knowing its molar mass allows us to calculate the amount of NaCl needed to react completely with a specific amount of another reactant.
2. Solution Preparation
In preparing solutions with specific concentrations, molar mass is essential. Chemists and biologists frequently work with molar solutions (M), which are defined as the number of moles of solute per liter of solution. Knowing the molar mass of NaCl allows precise preparation of solutions with known molar concentrations. This accuracy is critical in experiments and applications where precise concentrations are necessary.
3. Determining the Purity of NaCl Samples
The molar mass of NaCl can be used to determine the purity of a sample of sodium chloride. By carefully measuring the mass of a sample and then determining the number of moles using its molar mass, we can calculate the percentage purity of the sample. Any deviation from the expected molar mass suggests the presence of impurities.
4. Material Science and Engineering
In materials science and engineering, the molar mass of NaCl plays a role in understanding its crystal structure and physical properties. The size and arrangement of ions in the crystal lattice are directly related to the molar mass and contribute to properties like melting point, density, and solubility.
5. Biochemical and Physiological Processes
Sodium chloride plays a vital role in numerous biological processes. Understanding its molar mass is important for determining the concentration of sodium and chloride ions in biological fluids, such as blood and extracellular fluids. These concentrations are critical for maintaining proper osmotic balance and nerve impulse transmission. Deviations from normal concentrations can indicate physiological problems.
6. Industrial Applications
Many industrial processes utilize sodium chloride, and precise calculations involving its molar mass are crucial for efficiency and product quality. For example, in the production of chlorine gas and sodium hydroxide through electrolysis of brine (a solution of NaCl in water), molar mass is essential for optimizing the process and achieving the desired yields.
Advanced Concepts and Considerations
While the basic calculation of molar mass for NaCl is straightforward, there are some advanced considerations.
Isotopic Variations and Average Atomic Weight
The atomic weights used in calculations are average values, accounting for the natural abundance of different isotopes of sodium and chlorine. The isotopic composition can vary slightly depending on the source of the NaCl, which can lead to minor variations in the calculated molar mass. For highly precise work, the exact isotopic composition of the sample should be considered.
Hydrates and Other Forms of Sodium Chloride
Sodium chloride can exist in different forms, such as hydrates, where water molecules are incorporated into the crystal structure. The molar mass of a hydrated form of NaCl would be higher than the anhydrous form due to the additional mass of the water molecules. It is crucial to specify the form of sodium chloride (anhydrous or hydrated) when reporting its molar mass.
Conclusion: The Importance of Accurate Molar Mass Determination
The molar mass of sodium chloride, while seemingly a simple calculation, is a cornerstone concept in chemistry and has far-reaching implications in diverse fields. Accurate determination of molar mass is crucial for precise stoichiometric calculations, solution preparation, purity assessments, and understanding the physical and biological properties of this vital compound. The importance of considering isotopic variations and hydrated forms emphasizes the need for careful attention to detail in molar mass calculations, ensuring accurate results in various scientific and industrial applications. The seemingly simple number, 58.44 g/mol, represents a fundamental understanding of the composition and behavior of a compound that is essential to our daily lives.
Latest Posts
Latest Posts
-
What Is The Lcm For 7 And 9
Mar 17, 2025
-
44 Is 25 Of What Number
Mar 17, 2025
-
Finding Average Velocity Over An Interval
Mar 17, 2025
-
How Many Cm In 7 5 Inches
Mar 17, 2025
-
What Is The Fraction For 8
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Sodium Chloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
