What Are The Atoms That Make Up Carbohydrates
listenit
Apr 01, 2025 · 6 min read
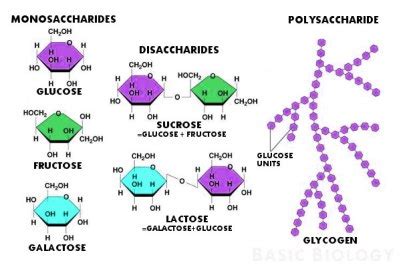
Table of Contents
What Are the Atoms That Make Up Carbohydrates? A Deep Dive into Carbohydrate Chemistry
Carbohydrates, often the first source of energy that comes to mind, are ubiquitous in our diet and crucial for life itself. But what exactly are they at a fundamental level? Understanding their atomic composition is key to grasping their properties and biological functions. This article will delve deep into the elemental makeup of carbohydrates, exploring their structure and how different arrangements of atoms lead to the diverse range of carbohydrates found in nature.
The Building Blocks: Carbon, Hydrogen, and Oxygen
At their core, carbohydrates are composed of just three elements: carbon (C), hydrogen (H), and oxygen (O). This simple elemental composition belies the incredible complexity and diversity found within the carbohydrate family. The ratio of hydrogen to oxygen atoms is typically 2:1, mirroring that found in water (H₂O), although this isn't a strict rule for all carbohydrates. This 2:1 ratio is reflected in the general formula for carbohydrates: (CH₂O)ₙ, where 'n' represents the number of carbon atoms in the molecule.
Carbon's Central Role
Carbon is the backbone of all organic molecules, including carbohydrates. Its unique ability to form four covalent bonds allows it to create long chains, branched structures, and ring formations, crucial for the diversity of carbohydrate structures. These carbon chains form the foundation upon which hydrogen and oxygen atoms are attached. The arrangement of these atoms and the presence of functional groups significantly influence the carbohydrate's properties and function.
Hydrogen and Oxygen: Contributing to Structure and Reactivity
Hydrogen and oxygen atoms, while not the primary structural component, are essential for defining the carbohydrate's properties and reactivity. The hydroxyl groups (-OH) attached to carbon atoms are crucial for the formation of glycosidic bonds, which link monosaccharides to form disaccharides, oligosaccharides, and polysaccharides. The presence and position of these hydroxyl groups significantly impact the solubility, reactivity, and overall behavior of carbohydrates.
Types of Carbohydrates and Their Atomic Arrangements
Carbohydrates are classified into different types based on their size and structure. This classification directly relates to the arrangement and number of carbon, hydrogen, and oxygen atoms.
1. Monosaccharides: The Simplest Carbohydrates
Monosaccharides are the simplest form of carbohydrates, also known as simple sugars. They are the building blocks for more complex carbohydrates. Common examples include glucose, fructose, and galactose. These monosaccharides typically contain three to seven carbon atoms arranged in a linear or cyclic structure. The specific arrangement of these atoms, including the position of hydroxyl groups and the presence of aldehyde or ketone functional groups, differentiates one monosaccharide from another. For example:
-
Glucose (C₆H₁₂O₆): A crucial source of energy for most organisms, glucose exists predominantly in a cyclic form, forming a six-membered ring. The position of the hydroxyl group on the first carbon atom differentiates between α-glucose and β-glucose, influencing the properties of polymers they form.
-
Fructose (C₆H₁₂O₆): A ketohexose (a six-carbon sugar with a ketone group), fructose also forms a ring structure, but it's a five-membered ring (furanose).
-
Galactose (C₆H₁₂O₆): An aldohexose (a six-carbon sugar with an aldehyde group), galactose is an isomer of glucose, differing only in the orientation of a hydroxyl group. These subtle differences in atomic arrangement lead to distinct properties and roles in biological processes.
2. Disaccharides: Two Monosaccharides Joined
Disaccharides are formed by the joining of two monosaccharides through a glycosidic bond. This bond is formed by a dehydration reaction where a water molecule is removed, linking the two monosaccharide units. Common disaccharides include:
-
Sucrose (table sugar): Composed of glucose and fructose.
-
Lactose (milk sugar): Composed of glucose and galactose.
-
Maltose (malt sugar): Composed of two glucose molecules.
The glycosidic bond’s specific location and configuration (α or β) influence the properties of the disaccharide. The atomic composition of a disaccharide is simply the sum of the atoms in its constituent monosaccharides minus the atoms lost during the dehydration reaction (two hydrogen atoms and one oxygen atom).
3. Oligosaccharides: Short Chains of Monosaccharides
Oligosaccharides contain 3-10 monosaccharide units linked together by glycosidic bonds. They are found in various foods and play roles in cell signaling and recognition. The atomic composition is a multiple of the monosaccharide units, minus the water molecules lost during the bond formation.
4. Polysaccharides: Long Chains of Monosaccharides
Polysaccharides are long chains consisting of hundreds or thousands of monosaccharide units linked by glycosidic bonds. These polymers are crucial for energy storage and structural support in living organisms. Examples include:
-
Starch (amylose and amylopectin): Primarily composed of glucose units, starch serves as an energy storage molecule in plants. The branching pattern of amylopectin, influenced by the arrangement of α-1,4 and α-1,6 glycosidic linkages, affects its digestibility.
-
Glycogen: The main energy storage polysaccharide in animals, glycogen is also composed of glucose units with extensive branching, allowing for rapid mobilization of glucose when needed.
-
Cellulose: A structural polysaccharide found in plant cell walls, cellulose consists of glucose units linked by β-1,4 glycosidic bonds. This different linkage creates a linear, rigid structure resistant to digestion by most animals. The linear arrangement and the beta linkage give cellulose its strength.
The atomic composition of polysaccharides is a multiple of the monosaccharide units, with water molecules lost during the formation of numerous glycosidic bonds. The specific arrangement of these units significantly impacts the physical and chemical properties of the polysaccharide.
Beyond the Basic Formula: Functional Groups and Isomerism
While the general formula (CH₂O)ₙ provides a simplified representation, a complete understanding requires considering the arrangement of atoms and the presence of functional groups. Several aspects contribute to carbohydrate diversity:
-
Isomerism: Carbohydrates can exhibit isomerism, where molecules have the same chemical formula but different structural arrangements. This leads to different properties and functions, as seen in glucose, fructose, and galactose (all C₆H₁₂O₆ but with different structures).
-
Functional Groups: The presence of hydroxyl (-OH), aldehyde (-CHO), and ketone (=O) functional groups significantly influences the reactivity and solubility of carbohydrates. These groups participate in chemical reactions, such as the formation of glycosidic bonds and oxidation-reduction reactions.
-
Anomeric Carbon: In cyclic monosaccharides, the carbon atom involved in the ring closure (the anomeric carbon) can have two different configurations (α and β), influencing the properties of the carbohydrate and the type of glycosidic bonds it can form.
-
Chirality: Many carbohydrates contain chiral centers (carbon atoms with four different substituents). The arrangement of substituents around these chiral centers determines the stereochemistry of the molecule, which affects its biological activity and interactions with enzymes.
Importance of Carbohydrate Structure and Function
The precise atomic arrangement and the resulting structural features of carbohydrates are directly linked to their biological functions:
-
Energy Source: The readily available energy stored in the bonds of carbohydrates fuels cellular processes. Glucose is the primary energy source for many organisms.
-
Energy Storage: Starch in plants and glycogen in animals store excess glucose for later use.
-
Structural Support: Cellulose provides structural rigidity to plant cell walls, while chitin forms the exoskeletons of arthropods.
-
Cell Recognition and Signaling: Oligosaccharides attached to proteins and lipids play crucial roles in cell-cell recognition, immune responses, and other cellular processes.
Conclusion: A Diverse Family Built on Simple Elements
Carbohydrates, despite being composed of only carbon, hydrogen, and oxygen, represent an incredibly diverse class of biomolecules. Their structural complexity arises from the different ways these atoms can be arranged, forming linear and cyclic structures, with variations in glycosidic linkages and functional groups. Understanding the fundamental atomic composition and the resulting structural features is crucial for appreciating the biological significance of carbohydrates in energy metabolism, structural support, and cellular communication. The seemingly simple formula (CH₂O)ₙ masks a world of intricate chemistry that sustains life itself.
Latest Posts
Latest Posts
-
24 To The Power Of 2
Apr 02, 2025
-
Lowest Common Multiple Of 14 And 35
Apr 02, 2025
-
What Is The Limit Of A Constant
Apr 02, 2025
-
X 3 2x 2 X 2 0
Apr 02, 2025
-
1 3 Divided By 1 6 As A Fraction
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are The Atoms That Make Up Carbohydrates . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
