What Are Monomers Of Nucleic Acids
listenit
Mar 24, 2025 · 6 min read
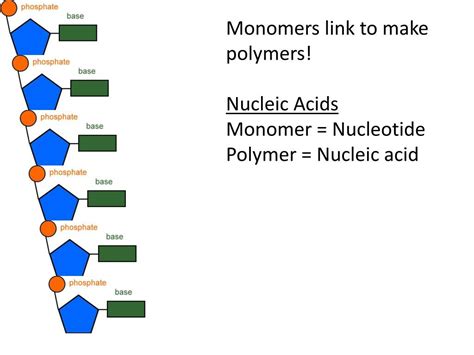
Table of Contents
What Are the Monomers of Nucleic Acids? A Deep Dive into Nucleotides
Nucleic acids, the fundamental building blocks of life, are responsible for storing and transmitting genetic information. These crucial biomolecules come in two primary forms: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Understanding their structure is paramount to grasping their function, and that understanding begins with their monomers: nucleotides. This article will delve into the intricate world of nucleotides, exploring their structure, composition, and the vital roles they play in the biological processes of all living organisms.
The Building Blocks: Nucleotides Explained
Nucleotides are the monomers, or individual units, that link together to form the long polymer chains of DNA and RNA. They're not simply simple units, however; each nucleotide is a complex molecule comprised of three essential components:
1. A Pentose Sugar: The Backbone's Foundation
The backbone of a nucleic acid is formed by a sugar molecule. In DNA, this is deoxyribose, a five-carbon sugar (pentose) lacking an oxygen atom on the 2' carbon. RNA, on the other hand, uses ribose, a pentose sugar with a hydroxyl group (-OH) attached to the 2' carbon. This seemingly small difference has significant implications for the structure and stability of the two nucleic acids. The absence of the hydroxyl group in deoxyribose contributes to DNA's greater stability, making it ideal for long-term storage of genetic information. The presence of the hydroxyl group in ribose makes RNA more reactive and less stable, reflecting its often transient roles in gene expression.
2. A Nitrogenous Base: The Information Carrier
Attached to the 1' carbon of the pentose sugar is a nitrogenous base. These bases are heterocyclic organic molecules containing nitrogen atoms. They are categorized into two main groups based on their structure:
-
Purines: These are double-ring structures, composed of a six-membered ring fused to a five-membered ring. The purines found in nucleic acids are adenine (A) and guanine (G).
-
Pyrimidines: These are single-ring, six-membered structures. The pyrimidines in nucleic acids are cytosine (C), thymine (T), and uracil (U). Note that thymine is found only in DNA, while uracil is found only in RNA. This difference is another key distinction between the two nucleic acids.
The specific sequence of these nitrogenous bases along the nucleic acid chain encodes the genetic information. The order of A, T, C, and G in DNA, or A, U, C, and G in RNA, determines the genetic code that dictates the synthesis of proteins and other essential molecules.
3. Phosphate Group: Linking the Units
The third component of a nucleotide is a phosphate group (PO₄³⁻). This negatively charged group is attached to the 5' carbon of the pentose sugar. The phosphate group plays a crucial role in linking nucleotides together to form the polynucleotide chain. The 5' phosphate of one nucleotide forms a phosphodiester bond with the 3' hydroxyl group of the adjacent nucleotide, creating the characteristic sugar-phosphate backbone of DNA and RNA. This creates a directionality to the chain, often referred to as the 5' to 3' direction.
Nucleotide Variations and Their Functions
While the basic structure of a nucleotide is consistent, variations exist, particularly in the number of phosphate groups attached to the sugar. These variations play significant roles in various cellular processes:
-
Nucleoside Monophosphates (NMPs): These contain a single phosphate group attached to the 5' carbon of the sugar. They are the basic building blocks of nucleic acids. Examples include adenosine monophosphate (AMP), guanosine monophosphate (GMP), cytidine monophosphate (CMP), thymidine monophosphate (TMP), and uridine monophosphate (UMP).
-
Nucleoside Diphosphates (NDPs): These contain two phosphate groups linked together. NDPs are crucial intermediates in many metabolic pathways, including energy transfer. Examples include adenosine diphosphate (ADP) and guanosine diphosphate (GDP).
-
Nucleoside Triphosphates (NTPs): These contain three phosphate groups. NTPs are high-energy molecules that provide the energy needed for many cellular processes, particularly in the synthesis of DNA and RNA. The most well-known examples are adenosine triphosphate (ATP), the primary energy currency of the cell, and guanosine triphosphate (GTP), involved in protein synthesis and signal transduction. Other examples include CTP, TTP, and UTP. These triphosphates are crucial for the polymerization reactions during DNA and RNA synthesis. The energy released from the hydrolysis of the high-energy phosphate bonds fuels the formation of the phosphodiester bonds that link nucleotides together.
Beyond the Basics: Specialized Nucleotides
The world of nucleotides extends beyond the simple monomers discussed above. Many specialized nucleotides play essential roles in cellular processes:
-
Cyclic AMP (cAMP): A derivative of AMP, cAMP is a crucial second messenger involved in signal transduction pathways, relaying signals from hormones and other extracellular stimuli to intracellular targets.
-
Cyclic GMP (cGMP): Similar to cAMP, cGMP is another important second messenger involved in various cellular processes, including vasodilation and vision.
-
Coenzyme A (CoA): While not strictly a nucleotide, CoA contains a nucleotide component (adenosine 3'-phosphate 5'-diphosphate) and plays a critical role in metabolism, particularly in the transfer of acetyl groups in the citric acid cycle.
-
NAD+ and NADP+: These are coenzymes crucial for redox reactions (oxidation-reduction reactions) in metabolism, acting as electron carriers. They contain nicotinamide, a nitrogenous base similar to those found in nucleotides.
-
FAD and FMN: These flavin coenzymes also participate in redox reactions, acting as electron carriers in metabolic pathways. They are derived from riboflavin (vitamin B2) and have structural similarities to nucleotides.
The Significance of Nucleotide Structure and Function
The precise structure of nucleotides, with their specific sugar, base, and phosphate components, is directly related to their function. The differences between DNA and RNA, stemming primarily from the difference between deoxyribose and ribose, contribute to their distinct roles in the cell. The high-energy bonds in NTPs power crucial cellular processes, while the sequence of bases in DNA and RNA encodes the genetic blueprint of life. The unique properties of specialized nucleotides highlight their diverse involvement in a wide range of cellular activities.
Conclusion: The Central Role of Nucleotides
In conclusion, nucleotides are far more than simple building blocks; they are versatile molecules with diverse roles crucial to the functioning of all living organisms. From their role as the monomers of DNA and RNA, carrying the genetic code, to their involvement as energy carriers and signaling molecules, nucleotides represent a fundamental level of biological organization and complexity. Understanding their structure, composition, and function is fundamental to understanding the mechanisms of life itself. Further research continuously reveals new intricacies and functions of these remarkable molecules, solidifying their position as central players in the biological world.
Latest Posts
Latest Posts
-
What Is 10 Minutes In Hours
Mar 26, 2025
-
What Is The Greatest Common Factor Of 12 And 15
Mar 26, 2025
-
What Is Oxidized And Reduced In Cellular Respiration
Mar 26, 2025
-
Chemical Reaction Of Iron And Oxygen
Mar 26, 2025
-
6 Pints Equals How Many Quarts
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Are Monomers Of Nucleic Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
