What Are Columns On The Periodic Table Called
listenit
Mar 27, 2025 · 6 min read
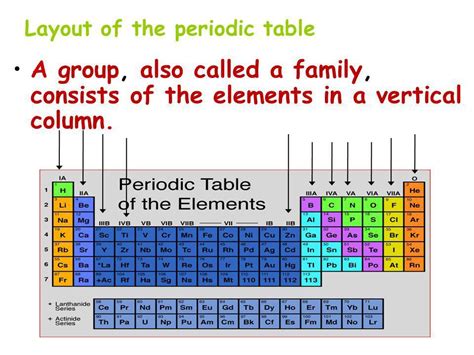
Table of Contents
- What Are Columns On The Periodic Table Called
- Table of Contents
- What Are Columns on the Periodic Table Called? Understanding Groups and Their Properties
- Defining Groups: Vertical Columns of Shared Traits
- The Significance of Outer Electrons
- Exploring Key Groups and Their Properties
- Group 1: Alkali Metals
- Group 2: Alkaline Earth Metals
- Group 17: Halogens
- Group 18: Noble Gases
- Transition Metals: A Unique Category
- Key Characteristics of Transition Metals:
- Understanding Group Trends: Periodicity in Action
- Key Periodic Trends:
- The Importance of Groups in Various Fields
- Conclusion: Groups—The Foundation of Chemical Understanding
- Latest Posts
- Latest Posts
- Related Post
What Are Columns on the Periodic Table Called? Understanding Groups and Their Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While rows are called periods, the columns are known as groups or families. Understanding the difference and the significance of these groupings is crucial for comprehending the behavior and reactivity of elements. This comprehensive guide delves into the intricacies of groups on the periodic table, exploring their characteristics, trends, and importance in various scientific fields.
Defining Groups: Vertical Columns of Shared Traits
Groups, the vertical columns of the periodic table, represent elements that share similar outer electron shell configurations. This similarity in electronic structure directly influences their chemical properties, resulting in strikingly similar behavior in reactions. For instance, elements within a single group often exhibit comparable electronegativity, ionization energy, and oxidation states. This shared characteristic is the fundamental reason why elements within a group are considered a 'family'. They react in similar ways, forming compounds with predictable properties.
The Significance of Outer Electrons
The outermost electron shell, also known as the valence shell, plays a pivotal role in determining an element's reactivity. Elements within the same group possess the same number of valence electrons, leading to their shared chemical behavior. It's the interaction of these valence electrons that dictates how an element will bond with other elements, forming molecules and compounds. Understanding this electron configuration is key to predicting an element's chemical reactivity.
Exploring Key Groups and Their Properties
The periodic table comprises 18 groups, each possessing unique characteristics. Let's delve into some of the most notable groups:
Group 1: Alkali Metals
The alkali metals, comprising lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), are highly reactive metals. Their single valence electron readily participates in chemical reactions, leading to the formation of +1 ions. They react vigorously with water, producing hydrogen gas and a metal hydroxide. Their reactivity increases as you move down the group due to the increasing atomic size and decreasing ionization energy.
Key Properties of Alkali Metals:
- Highly reactive: Readily lose one electron to form +1 ions.
- Low density: They are soft and can be easily cut with a knife.
- Low melting and boiling points: Compared to other metals.
- Good conductors of heat and electricity: Due to their loosely held valence electrons.
Group 2: Alkaline Earth Metals
The alkaline earth metals—beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra)—are also highly reactive, though less so than the alkali metals. They possess two valence electrons, readily forming +2 ions. They are harder and denser than the alkali metals and have higher melting and boiling points.
Key Properties of Alkaline Earth Metals:
- Reactive: Lose two electrons to form +2 ions, less reactive than alkali metals.
- Higher density and melting points: Than alkali metals.
- Essential for biological processes: Calcium and magnesium are crucial for various biological functions.
Group 17: Halogens
Halogens—fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)—are highly reactive non-metals. They have seven valence electrons, readily gaining one electron to form -1 ions. They are potent oxidizing agents, readily accepting electrons from other elements. Their reactivity decreases as you move down the group.
Key Properties of Halogens:
- Highly reactive nonmetals: Easily gain one electron to form -1 ions.
- Diatomic molecules: Exist as diatomic molecules (e.g., F₂, Cl₂).
- Varied physical states: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid at room temperature.
- Used in various applications: From disinfectants (chlorine) to pharmaceuticals (iodine).
Group 18: Noble Gases
Noble gases—helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn)—are exceptionally unreactive elements. They have a full valence shell (eight electrons, except for helium with two), making them very stable and resistant to forming chemical bonds. This inertness is a defining characteristic.
Key Properties of Noble Gases:
- Inert: Extremely unreactive due to their full valence electron shells.
- Colorless and odorless gases: At standard temperature and pressure.
- Used in various applications: Helium in balloons, neon in lighting, argon in welding.
Transition Metals: A Unique Category
The transition metals occupy the central block of the periodic table. They are characterized by their partially filled d orbitals, which results in variable oxidation states and a wide range of chemical properties. Unlike the main group elements, their properties are not as easily predicted based solely on their group number.
Key Characteristics of Transition Metals:
- Variable oxidation states: Can exhibit multiple oxidation states, leading to diverse chemical behavior.
- Formation of colored compounds: Many transition metal compounds exhibit vibrant colors.
- Catalytic activity: Many transition metals and their compounds act as catalysts in various chemical reactions.
- Magnetic properties: Some transition metals and their compounds exhibit magnetic properties.
Understanding Group Trends: Periodicity in Action
The periodic table's arrangement is not arbitrary. It reflects the periodic trends in elemental properties, meaning that certain properties exhibit a pattern as you move across a period or down a group. Understanding these trends allows for predictions about the chemical behavior of elements.
Key Periodic Trends:
- Electronegativity: The tendency of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period and decreases down a group.
- Ionization Energy: The energy required to remove an electron from an atom. Ionization energy generally increases across a period and decreases down a group.
- Atomic Radius: The size of an atom. Atomic radius generally decreases across a period and increases down a group.
- Metallic Character: The tendency of an element to lose electrons and form positive ions. Metallic character generally decreases across a period and increases down a group.
The Importance of Groups in Various Fields
The understanding and application of group properties are crucial in various scientific and technological fields:
- Chemistry: Predicting chemical reactions, synthesizing new compounds, and understanding chemical bonding.
- Materials Science: Designing new materials with specific properties, such as strength, conductivity, or reactivity.
- Biochemistry: Understanding the roles of elements in biological systems, such as the importance of calcium in bone structure.
- Medicine: Developing new drugs and therapies based on the properties of specific elements.
- Engineering: Selecting appropriate materials for various applications based on their properties, like corrosion resistance or strength.
Conclusion: Groups—The Foundation of Chemical Understanding
The columns of the periodic table, known as groups or families, represent a fundamental organizational principle that reflects the periodic recurrence of chemical properties. The shared electron configuration within a group determines its unique characteristics and reactivity. Understanding the properties of individual groups and the periodic trends within the table is crucial for comprehending chemical reactions, designing new materials, and advancing various scientific fields. From the highly reactive alkali metals to the inert noble gases, each group offers a unique window into the fascinating world of chemistry and its applications. The periodic table's structure, with its emphasis on groups and periods, provides a powerful tool for predicting and understanding the behaviour of the elements. Mastering this organization is a cornerstone of chemical literacy.
Latest Posts
Latest Posts
-
What Is 68 In Fraction Form
Mar 30, 2025
-
What Is 38 Out Of 40 As A Percentage
Mar 30, 2025
-
What Is The Value Of X In The Figure
Mar 30, 2025
-
1 3 4 Plus 1 3 4
Mar 30, 2025
-
A Car Advertisement States That A Certain
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Are Columns On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
