Water Is Always A Product In What Type Of Reaction
listenit
Mar 14, 2025 · 6 min read
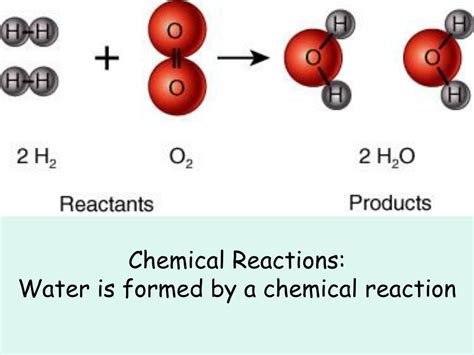
Table of Contents
Water is Always a Product in What Type of Reaction?
Water, the elixir of life, plays a crucial role in countless chemical reactions. Understanding its presence as a product can unlock deeper insights into various chemical processes. This comprehensive guide will delve into the types of reactions where water is invariably formed, exploring the underlying mechanisms and providing illustrative examples. We'll also touch upon the significance of water's formation in these reactions, highlighting its implications in diverse fields.
Acid-Base Reactions (Neutralization Reactions): The Primary Source of Water Formation
The most common scenario where water is a product is in acid-base reactions, also known as neutralization reactions. These reactions involve the reaction between an acid and a base, resulting in the formation of a salt and water. The hallmark of these reactions is the combination of hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base to form water (H₂O).
Understanding the Mechanism
The fundamental principle behind water formation in acid-base reactions lies in the neutralization of opposing charges. Acids donate protons (H⁺), while bases accept protons or donate hydroxide ions (OH⁻). When an acid and a base react, the H⁺ ions from the acid and the OH⁻ ions from the base combine to form a neutral molecule—water.
Example:
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example:
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
Here, the hydrogen ion (H⁺) from HCl combines with the hydroxide ion (OH⁻) from NaOH to produce water (H₂O). The remaining ions, Na⁺ and Cl⁻, form the salt sodium chloride (NaCl).
Different Types of Acid-Base Reactions
The production of water isn't limited to strong acid-strong base reactions. Weak acids and weak bases also react to form water, although the reaction may be less complete. Furthermore, acid-base reactions can occur in non-aqueous solvents, yet water often remains a product even under these altered conditions.
Combustion Reactions: Water as a Byproduct of Oxidation
Combustion reactions involve the rapid reaction of a substance with an oxidant, usually oxygen (O₂), producing heat and light. When organic compounds containing hydrogen (e.g., hydrocarbons, alcohols, carbohydrates) undergo combustion in the presence of sufficient oxygen, water is always formed as a byproduct.
The Role of Hydrogen in Combustion
The hydrogen atoms in the organic fuel react with the oxygen to form water. The carbon atoms react with oxygen to form carbon dioxide (CO₂). Therefore, the presence of hydrogen in the fuel is essential for water formation during combustion.
Example:
The complete combustion of methane (CH₄), the primary component of natural gas, illustrates this:
CH₄ (g) + 2O₂ (g) → CO₂ (g) + 2H₂O (g)
Here, the four hydrogen atoms from methane combine with two oxygen molecules to produce two molecules of water.
Incomplete Combustion and Water Formation
It is important to note that incomplete combustion, which occurs when there is insufficient oxygen, can produce carbon monoxide (CO) instead of carbon dioxide (CO₂). However, even under incomplete combustion, water usually remains a product, although the amount might be slightly less compared to complete combustion.
Hydration Reactions: Adding Water to a Molecule
Hydration reactions involve the addition of water to a molecule, often resulting in the breaking of a double or triple bond and the formation of new functional groups. While water isn't always solely produced, it is a crucial reactant that is incorporated into the final product's structure. Consider the hydration of an alkene to form an alcohol. While water itself isn't generated anew, it’s chemically integrated.
Hydration of Alkenes
The hydration of alkenes, a classic example of an addition reaction, involves the addition of water across the carbon-carbon double bond. This reaction typically requires an acid catalyst (such as sulfuric acid) to facilitate the process.
Example:
The hydration of ethene (C₂H₄) to form ethanol (C₂H₅OH):
C₂H₄ (g) + H₂O (l) → C₂H₅OH (l)
Although water is a reactant here, it becomes chemically integrated into the ethanol molecule, significantly altering its properties. The overall process is still considered a hydration reaction, showing water's significant role even if it's not solely produced.
Hydrolysis Reactions: Breaking Bonds with Water
Hydrolysis reactions are the reverse of condensation reactions. They involve the breaking of a chemical bond by the addition of water. Water acts as a reactant, splitting the molecule into two or more smaller molecules. The products frequently include a hydroxyl group (-OH) on one fragment and a hydrogen atom on the other.
Hydrolysis of Esters
Esters, often found in fats and oils, undergo hydrolysis in the presence of an acid or base catalyst to produce carboxylic acids and alcohols.
Example:
The acid-catalyzed hydrolysis of ethyl acetate:
CH₃COOCH₂CH₃ (l) + H₂O (l) → CH₃COOH (aq) + CH₃CH₂OH (aq)
In this case, water acts as a reactant, splitting the ester molecule into acetic acid and ethanol. While water is consumed, its presence is paramount for the reaction to proceed. The products are formed through bond breakage facilitated by the addition of a water molecule.
Synthesis Reactions and Water as a Byproduct: Unexpected Formation
While not as common as in acid-base or combustion reactions, certain synthesis reactions can generate water as a byproduct. This usually occurs when two molecules react, and the elimination of a water molecule leads to the formation of a new, larger molecule.
Condensation Reactions: Water as a Leaving Group
Condensation reactions are a prime example where water is a byproduct of the synthesis process. These reactions involve the joining of two molecules through the removal of a water molecule. The water molecule is formed by the combination of a hydroxyl group (-OH) from one molecule and a hydrogen atom from the other.
Example:
The formation of a peptide bond between two amino acids is a classic example of a condensation reaction:
Amino acid 1 + Amino acid 2 → Dipeptide + H₂O
Here, the removal of a water molecule allows the formation of a peptide bond between the two amino acids, resulting in a dipeptide. The water molecule is a byproduct of this synthetic process.
The Significance of Water Formation in Reactions
The formation of water in chemical reactions carries significant implications across various disciplines:
-
Energy Production: Combustion reactions, which produce water, are crucial for energy generation in power plants and internal combustion engines.
-
Industrial Processes: Many industrial processes rely on acid-base reactions or hydrolysis for the synthesis of various chemicals, resulting in water as a byproduct.
-
Environmental Science: Water formation in combustion reactions is essential for understanding atmospheric chemistry and the impact of greenhouse gases.
-
Biological Systems: Water plays a vital role in biological systems, acting as a solvent and a reactant in numerous biochemical processes. The formation and consumption of water are intimately linked with metabolic pathways.
-
Analytical Chemistry: The quantification of water produced in a reaction can be used to determine the amount of reactants involved, providing valuable information for analytical purposes.
Conclusion: Water's Ubiquitous Role
Water's formation in chemical reactions, especially acid-base and combustion reactions, is a widespread phenomenon. Understanding the mechanisms and significance of water production provides a crucial foundation for comprehending a vast array of chemical and biological processes. From energy generation to environmental monitoring and biological functions, the role of water is ubiquitous, highlighting its importance in shaping the world around us. Further exploration into specific reactions will only reveal further instances of its crucial involvement, reinforcing its central role in the chemical universe.
Latest Posts
Latest Posts
-
What Is The Density Of Water In G Ml
Mar 15, 2025
-
Whats The Gcf Of 12 And 30
Mar 15, 2025
-
How Many Inches Is 4 5 Feet
Mar 15, 2025
-
X 3 4x 2 4x 16
Mar 15, 2025
-
How Do I Calculate Average Atomic Mass
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Water Is Always A Product In What Type Of Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
