Two Different Isotopes Of An Element Have Different
listenit
Mar 21, 2025 · 6 min read
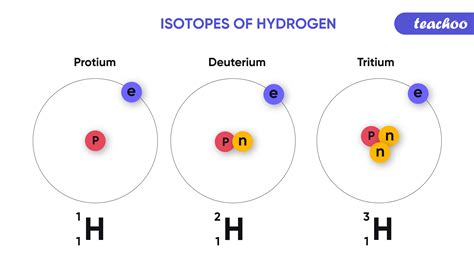
Table of Contents
Two Different Isotopes of an Element Have Different… Properties!
Isotopes, those fascinating variations of the same element, often get overlooked in basic chemistry discussions. However, understanding their differences is crucial for comprehending a wide range of phenomena, from nuclear energy to geological dating and even medical applications. This article delves deep into the fascinating world of isotopes, explaining why two isotopes of the same element exhibit different properties, despite sharing the same atomic number.
What are Isotopes?
Before we explore their differences, let's establish a clear understanding of what isotopes are. Isotopes are atoms of the same element that possess the same number of protons (defining the element) but differ in the number of neutrons. Remember, the atomic number of an element is determined solely by the number of protons in its nucleus. Neutrons, on the other hand, reside in the nucleus alongside protons and contribute to the atom's mass but not its chemical identity.
For example: Carbon-12 (¹²C) and Carbon-14 (¹⁴C) are isotopes of carbon. Both have 6 protons (hence they are both carbon), but ¹²C has 6 neutrons, while ¹⁴C has 8 neutrons. This difference in neutron number significantly impacts their properties.
The Key Difference: Mass Number and Nuclear Stability
The most obvious difference between isotopes lies in their mass number. The mass number is the sum of protons and neutrons in an atom's nucleus. Since isotopes vary in neutron number, their mass numbers also differ. This difference in mass directly influences several properties:
1. Mass and Density:
The increased neutron number in heavier isotopes results in a higher atomic mass. This difference, while seemingly small at the atomic level, becomes significant when considering macroscopic quantities of the element. For instance, a sample of heavy water (containing deuterium, ²H, an isotope of hydrogen) will be denser than ordinary water (containing ¹H). This density difference is exploited in various separation techniques.
2. Nuclear Stability and Radioactivity:
Perhaps the most significant difference between isotopes stems from their nuclear stability. The ratio of protons to neutrons within the nucleus profoundly influences an isotope's stability. Some isotope combinations are stable, while others are unstable and undergo radioactive decay. This decay involves emitting particles or energy to achieve a more stable nuclear configuration.
Radioactive isotopes, such as Carbon-14, are widely used in:
- Radiocarbon dating: By measuring the remaining ¹⁴C in organic materials, scientists can estimate their age.
- Medical imaging and treatment: Radioactive isotopes like iodine-131 are used in diagnosing and treating thyroid disorders.
- Tracing chemical processes: Radioactive isotopes act as tracers in various chemical and biological processes, allowing researchers to track the movement and transformation of molecules.
Stable isotopes, on the other hand, do not undergo radioactive decay. They are essential components of numerous biological and geological processes. Their abundance varies naturally in different environments, making them valuable tools in:
- Paleoclimatology: Studying the isotopic ratios of elements in ancient ice cores and sediments provides insights into past climates.
- Forensic science: Isotope ratios in materials like hair and teeth can help trace individuals' geographic origins and diets.
- Environmental monitoring: Analyzing stable isotope ratios in water and soil can reveal pollution sources and ecological changes.
3. Physical Properties beyond Mass:
While mass is the most obvious difference, subtle variations in other physical properties can also exist. This is particularly true for isotopes with significantly differing neutron numbers. These variations may be related to:
- Melting point and boiling point: Small changes in vibrational frequencies within the molecular structure can lead to slight variations in these properties.
- Spectroscopic properties: Isotopes can exhibit slightly different spectral lines due to variations in their nuclear mass and spin. This difference is often used in isotope analysis techniques like mass spectrometry.
- Diffusion rates: Heavier isotopes diffuse slightly slower than lighter ones due to their greater mass. This difference is exploited in some isotope separation methods.
Why Do These Differences Occur?
The differences in properties between isotopes arise primarily from the differences in their nuclear mass and nuclear spin.
-
Nuclear Mass: The added mass from extra neutrons influences the kinetic energy of the atoms and molecules containing these isotopes, leading to variations in physical properties like diffusion rates and vibrational frequencies. The greater mass also affects the interactions between atoms, leading to subtle changes in properties like melting and boiling points.
-
Nuclear Spin: Neutrons and protons possess an intrinsic angular momentum called spin. The total nuclear spin, resulting from the combined spins of protons and neutrons, influences the atom's magnetic properties and interactions with external magnetic fields. This difference in nuclear spin can lead to subtle variations in spectroscopic properties.
Additionally, the difference in nuclear stability profoundly influences the chemical behavior of radioactive isotopes. Their decay processes release energy and particles, which can alter the chemical environment and induce different reactions compared to their stable counterparts.
Isotopes and Chemical Properties: The Subtlety
It's crucial to emphasize that while isotopes differ in physical properties, their chemical properties remain largely the same. This is because chemical properties are primarily determined by the electron configuration, which is dictated by the number of protons (atomic number). Since all isotopes of a given element have the same number of protons, they possess the same electron configuration and thus exhibit similar chemical behavior.
However, kinetic isotope effects can manifest. These are subtle differences in reaction rates due to the mass differences between isotopes. Heavier isotopes generally react slightly slower than lighter ones because their vibrational frequencies are lower, influencing the activation energy of reactions. These effects are often small but can be significant in specific reactions or under certain conditions.
Examples of Isotope Differences in Action
Let's look at some concrete examples:
-
Hydrogen Isotopes: Hydrogen has three isotopes: protium (¹H), deuterium (²H), and tritium (³H). Deuterium is twice as massive as protium, leading to noticeable differences in physical properties. Heavy water (D₂O) has a higher boiling point and density than ordinary water (H₂O). Tritium is radioactive and undergoes beta decay.
-
Carbon Isotopes: ¹²C is the most abundant and stable isotope of carbon. ¹⁴C, used in carbon dating, is radioactive with a half-life of about 5,730 years. The slight mass difference between ¹²C and ¹⁴C can influence biological processes, affecting the rates of certain metabolic reactions.
-
Uranium Isotopes: Uranium has several isotopes, with ²³⁵U and ²³⁸U being the most common. ²³⁵U is fissile (capable of undergoing nuclear fission), while ²³⁸U is not. This difference is fundamental to nuclear power generation and nuclear weaponry. The separation of these isotopes is a technologically challenging process.
Conclusion: A World of Isotopic Diversity
The differences between isotopes, though subtle in some cases, are profound in their implications. From the fundamental properties of matter to advancements in medicine, geology, and energy production, understanding isotopes is crucial. Their varied mass, nuclear stability, and resulting physical and subtle chemical differences provide a rich tapestry of possibilities and drive ongoing research across multiple scientific disciplines. Further investigation into the nuanced properties of isotopes promises exciting discoveries in the years to come.
Latest Posts
Latest Posts
-
What Is The Improper Fraction Of 2 1 2
Mar 28, 2025
-
What Are The Coordinates Of Point S
Mar 28, 2025
-
A Mixture In Which Substances Are Distributed Evenly
Mar 28, 2025
-
Y Square Root Of X 4
Mar 28, 2025
-
Differentiate Between Fermentation And Anaerobic Respiration
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Two Different Isotopes Of An Element Have Different . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
