The Sum Of The Protons And Neutrons In An Atom
listenit
Mar 17, 2025 · 6 min read
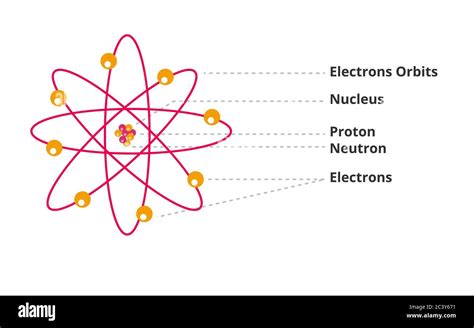
Table of Contents
The Sum of Protons and Neutrons in an Atom: Unveiling the Atomic Mass Number
The seemingly simple sum of protons and neutrons within an atom's nucleus holds the key to understanding a fundamental property: atomic mass number. This seemingly straightforward concept underpins numerous aspects of chemistry, physics, and nuclear science, impacting everything from the periodic table's organization to nuclear reactions. This comprehensive guide delves into the intricacies of atomic mass number, exploring its definition, calculation, significance, and applications.
Understanding the Atomic Nucleus: Protons and Neutrons
Before diving into the sum of protons and neutrons, let's establish a clear understanding of the atom's core components. The atom's nucleus, a tiny but incredibly dense region, houses two types of subatomic particles:
-
Protons: Positively charged particles, carrying a charge of +1. The number of protons in an atom's nucleus defines its atomic number, a unique identifier for each element on the periodic table. Hydrogen, for example, with one proton, has an atomic number of 1.
-
Neutrons: Electrically neutral particles, carrying no charge. While they don't contribute to the atom's overall charge, neutrons play a crucial role in stabilizing the nucleus and influencing an atom's mass.
The number of protons is constant for a given element. However, the number of neutrons can vary, leading to different isotopes of the same element.
Defining Atomic Mass Number (A): The Sum of Protons and Neutrons
The atomic mass number (A), also known as the mass number, is simply the sum of the number of protons and neutrons in an atom's nucleus. This is represented mathematically as:
A = Z + N
Where:
- A represents the atomic mass number.
- Z represents the atomic number (number of protons).
- N represents the number of neutrons.
The atomic mass number provides a whole-number approximation of an atom's mass, expressed in atomic mass units (amu). It's important to remember that this is an approximation because it doesn't account for the small mass differences between protons and neutrons, or the binding energy that holds the nucleus together.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element (same atomic number, Z) that have different numbers of neutrons (N), and therefore different atomic mass numbers (A). For instance, carbon (atomic number 6) has several isotopes:
- Carbon-12 (¹²C): 6 protons + 6 neutrons = atomic mass number 12
- Carbon-13 (¹³C): 6 protons + 7 neutrons = atomic mass number 13
- Carbon-14 (¹⁴C): 6 protons + 8 neutrons = atomic mass number 14
These isotopes exhibit similar chemical properties due to their identical number of protons and electrons, but they may differ in their physical properties (e.g., stability, radioactive decay). Carbon-14, for example, is a radioactive isotope used in radiocarbon dating.
Calculating Atomic Mass Number: Examples and Practice
Calculating the atomic mass number is straightforward once you know the number of protons and neutrons. Let's look at some examples:
Example 1: An atom has 17 protons and 18 neutrons. What is its atomic mass number?
A = Z + N = 17 + 18 = 35
Therefore, the atomic mass number is 35.
Example 2: An atom of oxygen has an atomic number of 8 and an atomic mass number of 16. How many neutrons does it have?
N = A - Z = 16 - 8 = 8
The oxygen atom has 8 neutrons.
Example 3: A potassium atom has 19 protons and 20 neutrons. What is its atomic symbol, including the mass number?
The atomic number 19 identifies the element as potassium (K). The atomic mass number is 19 + 20 = 39. Therefore, the symbol is ³⁹K.
These examples illustrate the simplicity of calculating the atomic mass number. This fundamental concept serves as a crucial building block for understanding more complex nuclear phenomena.
Significance of Atomic Mass Number in Chemistry and Physics
The atomic mass number holds significant importance across various scientific disciplines:
-
Periodic Table Organization: While the atomic number primarily determines an element's position on the periodic table, the atomic mass number helps differentiate isotopes of the same element.
-
Nuclear Reactions: Atomic mass numbers are essential in balancing nuclear equations, ensuring the conservation of mass and charge during nuclear reactions such as fission and fusion. Understanding how atomic mass numbers change during these processes allows scientists to predict the products of nuclear reactions.
-
Mass Spectrometry: Mass spectrometry techniques rely heavily on the atomic mass number to identify and quantify different isotopes within a sample. This application is valuable in various fields, including environmental monitoring, forensic science, and medical diagnostics.
-
Nuclear Stability: The ratio of neutrons to protons significantly influences the stability of an atom's nucleus. Certain neutron-to-proton ratios contribute to greater nuclear stability, while others lead to radioactive decay. The atomic mass number plays a critical role in understanding these stability patterns.
-
Radioactive Decay: Radioactive isotopes undergo decay, changing their atomic mass number and often transforming into different elements. Understanding the changes in atomic mass number during radioactive decay is crucial in many applications, including medical treatments and geological dating.
Applications of Atomic Mass Number: Real-World Examples
The atomic mass number's significance transcends theoretical concepts, finding practical applications in diverse fields:
-
Nuclear Medicine: Radioactive isotopes with specific atomic mass numbers are used in diagnostic imaging and cancer treatment. For example, Iodine-131 (¹³¹I) is used in thyroid cancer treatment, and Technetium-99m (⁹⁹mTc) is widely used in various diagnostic scans.
-
Radiocarbon Dating: Carbon-14 (¹⁴C), with its specific atomic mass number, plays a critical role in determining the age of organic materials. The decay rate of ¹⁴C allows scientists to estimate the time elapsed since an organism died.
-
Nuclear Power: Nuclear power plants utilize nuclear fission reactions, where the atomic mass numbers of isotopes like Uranium-235 (²³⁵U) are crucial in controlling the chain reaction and generating energy.
-
Nuclear Weaponry: Similar to nuclear power, the atomic mass numbers of isotopes like Plutonium-239 (²³⁹Pu) and Uranium-235 (²³⁵U) are critical factors in designing and understanding nuclear weapons.
-
Materials Science: The study of materials properties often involves investigating isotopes with different atomic mass numbers, leading to the development of new materials with enhanced characteristics.
Beyond Atomic Mass Number: Isotopic Abundance and Average Atomic Mass
While the atomic mass number provides a valuable approximation of an atom's mass, it's essential to consider isotopic abundance and average atomic mass for a more accurate representation.
-
Isotopic Abundance: This refers to the relative proportion of each isotope of an element found in nature. For example, carbon exists predominantly as ¹²C (approximately 98.9%) and ¹³C (approximately 1.1%).
-
Average Atomic Mass: This is a weighted average of the atomic masses of all naturally occurring isotopes of an element, considering their relative abundances. The average atomic mass is the value shown on the periodic table for each element and is crucial in various stoichiometric calculations.
Conclusion: The Fundamental Role of Atomic Mass Number
The sum of protons and neutrons, resulting in the atomic mass number, is a seemingly simple yet remarkably significant concept in atomic and nuclear science. Its applications extend across numerous disciplines, impacting our understanding of the elements, nuclear reactions, and the development of advanced technologies. From the organization of the periodic table to the precise dating of ancient artifacts and the harnessing of nuclear energy, the atomic mass number plays a fundamental role in shaping our scientific knowledge and technological advancements. A thorough understanding of this concept forms the cornerstone for exploring the complexities of the atomic world.
Latest Posts
Latest Posts
-
What Is 7 12 As A Decimal
Mar 17, 2025
-
Solve X 2 2x 2 0
Mar 17, 2025
-
Molar Mass Of Sodium Carbonate Decahydrate
Mar 17, 2025
-
What Is The Derivative Of Cosecant
Mar 17, 2025
-
How Many Inches Is 1 4 Of A Yard
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Sum Of The Protons And Neutrons In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
