The Smallest Unit Of An Element
listenit
Mar 17, 2025 · 6 min read
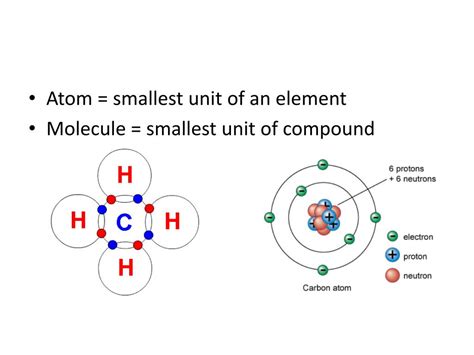
Table of Contents
The Smallest Unit of an Element: Delving into Atoms and Subatomic Particles
The quest to understand the fundamental building blocks of matter has captivated scientists for centuries. From ancient Greek philosophers pondering the nature of reality to modern physicists exploring the quantum realm, the search for the smallest unit of an element has driven groundbreaking discoveries and reshaped our understanding of the universe. While the simple answer might seem to be "the atom," the reality is far richer and more complex. This article delves deep into the fascinating world of atoms and their constituent subatomic particles, exploring their properties, behaviors, and significance in shaping our world.
Atoms: The Indivisible Building Blocks (or are they?)
For a long time, the atom was considered the smallest indivisible unit of an element. The word "atom" itself, derived from the Greek word "atomos," meaning "indivisible," reflects this historical perspective. Early models, like Dalton's atomic theory, envisioned atoms as solid, indivisible spheres. However, subsequent discoveries revolutionized our understanding, revealing that atoms are far from indivisible, possessing an intricate internal structure.
Exploring the Atomic Structure: A Journey into the Nucleus and Beyond
The modern understanding of the atom depicts a central nucleus, composed of positively charged protons and electrically neutral neutrons, surrounded by a cloud of negatively charged electrons. The nucleus is incredibly dense, containing almost all the atom's mass, while the electrons occupy a much larger volume, defining the atom's size. The number of protons in an atom's nucleus defines its atomic number, which uniquely identifies an element. Atoms of the same element always have the same number of protons.
Key Characteristics of Subatomic Particles:
- Protons (p+): Positively charged particles with a mass approximately 1836 times greater than an electron. The number of protons determines the element's identity.
- Neutrons (n0): Neutral particles with a mass slightly larger than a proton. Neutrons play a crucial role in stabilizing the nucleus and influencing the atom's isotope.
- Electrons (e-): Negatively charged particles with a mass significantly smaller than protons and neutrons. Electrons occupy specific energy levels or orbitals surrounding the nucleus and determine the atom's chemical properties.
Isotopes: Variations on a Theme
While the number of protons defines an element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes exhibit similar chemical properties but can have different physical properties, such as mass and radioactivity. For example, carbon-12 and carbon-14 are isotopes of carbon, differing in the number of neutrons. Carbon-14 is radioactive and is used in radiocarbon dating.
Beyond Atoms: Delving into Subatomic Particles
The discovery of the electron, proton, and neutron marked a significant step in our understanding of matter, but the story doesn't end there. Further research revealed that these particles are themselves composed of even smaller fundamental constituents called quarks and leptons.
Quarks: The Building Blocks of Protons and Neutrons
Protons and neutrons are not fundamental particles but are composed of three quarks each. There are six types of quarks: up, down, charm, strange, top, and bottom. Protons consist of two up quarks and one down quark, while neutrons are made of one up quark and two down quarks. The strong force, mediated by gluons, binds these quarks together within protons and neutrons.
Leptons: Including the Elusive Electron
Electrons belong to a family of fundamental particles called leptons. Leptons are fundamental particles that do not experience the strong force. Besides electrons, other leptons include muons and tau particles, each with its corresponding neutrino.
The Standard Model of Particle Physics: A Framework for Understanding
The Standard Model of Particle Physics provides a comprehensive framework for understanding the fundamental constituents of matter and their interactions. It incorporates quarks, leptons, and force-carrying particles like photons (electromagnetism), gluons (strong force), W and Z bosons (weak force), and the hypothetical graviton (gravity). The Standard Model has been incredibly successful in predicting and explaining numerous experimental results, but it's not without its limitations. It doesn't incorporate gravity and leaves several open questions about the universe’s composition, such as the nature of dark matter and dark energy.
The Quantum Realm: Where Intuition Fails
Understanding atoms and subatomic particles requires venturing into the realm of quantum mechanics. Quantum mechanics describes the behavior of matter at the atomic and subatomic levels, where classical physics fails. Concepts like wave-particle duality, quantum superposition, and uncertainty principle are essential for grasping the intricacies of the quantum world.
Wave-Particle Duality: The Enigma of Behavior
One of the most counterintuitive aspects of quantum mechanics is the wave-particle duality. Subatomic particles, like electrons, exhibit both wave-like and particle-like properties. They can behave as waves, exhibiting diffraction and interference patterns, yet they also interact like particles, having definite properties like mass and charge.
Quantum Superposition: Existing in Multiple States Simultaneously
Another mind-bending concept is quantum superposition. A quantum particle can exist in multiple states simultaneously until it is measured. For example, an electron can be in a superposition of multiple energy levels until a measurement forces it into a definite state. This is a critical aspect of quantum computing.
The Uncertainty Principle: Limits on Precision
The Heisenberg uncertainty principle states that it is impossible to simultaneously know both the position and momentum of a particle with perfect accuracy. The more precisely we know one, the less precisely we know the other. This fundamental limit arises from the quantum nature of particles and imposes constraints on our ability to make precise measurements at the subatomic level.
Implications and Applications: From Technology to Cosmology
The understanding of atoms and subatomic particles has profoundly impacted various fields, from technology to cosmology.
Technology: Powering Modern Advancements
Our modern technological landscape relies heavily on our understanding of atomic structure and behavior. Semiconductors, the foundation of modern electronics, leverage the quantum properties of electrons in silicon and other materials. Nuclear medicine utilizes radioactive isotopes for diagnosis and treatment. Laser technology is based on manipulating atomic transitions.
Cosmology: Unveiling the Universe's Secrets
The study of subatomic particles is also crucial for cosmology, the study of the universe's origin and evolution. The Big Bang theory, our current best explanation for the universe's origin, relies on our understanding of fundamental particles and their interactions in the early universe. The search for dark matter and dark energy, which constitute most of the universe's mass-energy, involves investigating exotic subatomic particles and their interactions.
Conclusion: An Ongoing Journey of Discovery
While the atom may have once been considered the smallest indivisible unit of an element, our understanding has evolved significantly. We now know that atoms are complex systems composed of subatomic particles governed by the laws of quantum mechanics. These particles, in turn, are made up of even more fundamental constituents, revealing a universe of astonishing complexity and elegance. The journey to understand the smallest unit of an element continues, driving ongoing research and pushing the boundaries of our knowledge. The quest remains a testament to human curiosity and the enduring pursuit of fundamental truths about the universe we inhabit. As we continue to explore the quantum realm, we can expect even more groundbreaking discoveries that will reshape our understanding of matter and the universe itself. The exploration of atoms and subatomic particles not only pushes the frontiers of science but also holds the potential for revolutionizing technologies and providing answers to some of the most profound questions humanity has ever posed.
Latest Posts
Latest Posts
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
-
What Is 10 To The Power Of 7
Mar 17, 2025
-
Lowest Common Multiple Of 4 And 10
Mar 17, 2025
-
Whats The Square Root Of 40
Mar 17, 2025
-
How To Convert Wavelength To Meters
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Smallest Unit Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
