The Particles Found In The Nucleus Of An Atom Are
listenit
Mar 23, 2025 · 7 min read
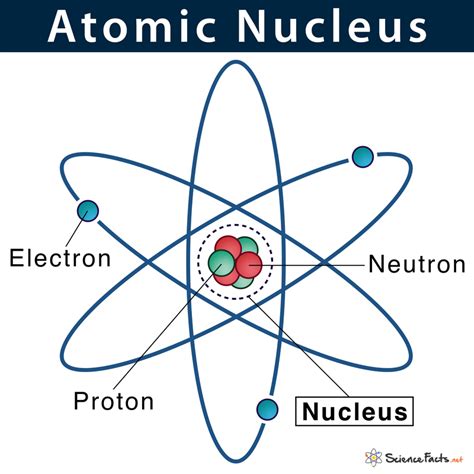
Table of Contents
The Particles Found in the Nucleus of an Atom: A Deep Dive
The atom, once considered the fundamental indivisible unit of matter, is now understood to be a complex system composed of subatomic particles. While the atom as a whole is electrically neutral, its nucleus, a tiny, dense core, houses the positively charged protons and the electrically neutral neutrons. Understanding the nature and properties of these particles is crucial to comprehending the behavior of matter and the forces that govern the universe. This article will delve deep into the fascinating world of nuclear particles, exploring their properties, interactions, and significance in various fields.
Protons: The Positive Charge Carriers
Protons are fundamental particles that carry a single positive electrical charge (+1e), where 'e' represents the elementary charge. Their mass is approximately 1,836 times that of an electron, contributing significantly to the overall mass of the atom's nucleus. The number of protons in an atom's nucleus defines its atomic number, a unique identifier that determines the element to which the atom belongs. For instance, an atom with one proton is hydrogen, an atom with six protons is carbon, and an atom with 92 protons is uranium.
Properties of Protons:
- Electric Charge: +1e
- Mass: Approximately 1.6726 × 10^-27 kg (or 1 atomic mass unit, amu)
- Spin: 1/2 (fermion)
- Composition: Composed of three quarks: two up quarks and one down quark.
- Stability: Protons are remarkably stable particles, with no evidence of spontaneous decay observed to date. This stability is crucial for maintaining the integrity of atomic nuclei.
Role of Protons in Chemical Reactions:
The number of protons directly dictates the chemical behavior of an atom. The outermost electrons, which are influenced by the positive charge of the protons in the nucleus, participate in chemical bonding and reactions. The strong positive charge of the protons holds the negatively charged electrons in their orbits, creating the structure of the atom. Changes in the number of protons result in a completely different element, fundamentally altering its chemical properties.
Neutrons: The Neutral Partners
Neutrons, as their name suggests, carry no net electrical charge (0e). Their mass is slightly larger than that of protons, approximately 1.6749 × 10^-27 kg (or slightly more than 1 amu). Unlike protons, the number of neutrons in an atom's nucleus can vary without changing the element. Atoms of the same element with different numbers of neutrons are called isotopes.
Properties of Neutrons:
- Electric Charge: 0e
- Mass: Approximately 1.6749 × 10^-27 kg (or slightly more than 1 amu)
- Spin: 1/2 (fermion)
- Composition: Composed of three quarks: one up quark and two down quarks.
- Stability: Free neutrons are unstable and undergo beta decay, transforming into a proton, an electron, and an antineutrino, with a half-life of about 10 minutes. However, neutrons within a stable atomic nucleus are generally stable.
Isotopes and Their Significance:
The presence of varying numbers of neutrons within the nucleus creates isotopes of the same element. Some isotopes are stable, while others are radioactive, undergoing decay processes that emit particles and energy. Radioactive isotopes have numerous applications, including medical imaging (e.g., PET scans), cancer therapy, and carbon dating in archaeology. The stability of an isotope depends on the balance between the strong nuclear force holding the nucleus together and the electromagnetic repulsion between the protons. Too many or too few neutrons can lead to nuclear instability and radioactive decay.
The Strong Nuclear Force: The Glue that Holds the Nucleus Together
The nucleus of an atom is an incredibly dense region of space, packed with positively charged protons that should, based on the principles of electromagnetism, repel each other strongly. However, the nucleus remains stable due to the strong nuclear force, one of the four fundamental forces in nature. This force is much stronger than the electromagnetic force at short distances, effectively overcoming the electrostatic repulsion between protons and binding the protons and neutrons together.
Properties of the Strong Nuclear Force:
- Short Range: The strong nuclear force is effective only over extremely short distances, approximately the size of the nucleus.
- Attractive Force: It acts as an attractive force between nucleons (protons and neutrons).
- Charge Independent: The force is largely independent of the electric charge of the nucleons, meaning it acts similarly on both protons and neutrons.
- Saturation: The strong force exhibits saturation, meaning that a nucleon interacts strongly with only a limited number of neighboring nucleons.
Importance of the Strong Nuclear Force:
The strong nuclear force is essential for the existence of stable atoms and hence, matter as we know it. Without this force, atomic nuclei would disintegrate due to the repulsive forces between protons. The balance between the strong nuclear force and the electromagnetic force determines the stability and structure of the nucleus.
Beyond Protons and Neutrons: A Glimpse into the Quark Model
Protons and neutrons are not fundamental particles themselves but are composed of even smaller constituents called quarks. Quarks are fundamental particles that interact via the strong nuclear force, mediated by gluons. There are six types, or "flavors," of quarks: up, down, charm, strange, top, and bottom. Protons and neutrons are each composed of three quarks bound together by gluons.
Quark Composition of Protons and Neutrons:
- Proton: Two up quarks and one down quark (uud)
- Neutron: One up quark and two down quarks (udd)
Gluons: The Mediators of the Strong Force:
Gluons are the fundamental force carriers of the strong interaction. They are massless particles that mediate the strong force between quarks, holding them together to form protons and neutrons. Gluons have a unique property called "self-interaction," meaning they can interact with each other, leading to complex dynamics within the nucleus.
Nuclear Stability and Radioactive Decay
The stability of an atomic nucleus depends on the delicate balance between the strong nuclear force and the electromagnetic force. Nuclei with an optimal ratio of protons to neutrons are generally stable. However, many nuclei are unstable, undergoing radioactive decay to achieve a more stable configuration. Radioactive decay involves the emission of particles and/or energy, transforming the nucleus into a different isotope or element.
Types of Radioactive Decay:
- Alpha Decay: Emission of an alpha particle (two protons and two neutrons)
- Beta Decay: Emission of a beta particle (an electron or a positron)
- Gamma Decay: Emission of a gamma ray (high-energy photon)
Significance of Radioactive Decay:
Radioactive decay has far-reaching consequences, influencing the composition of the earth's crust, powering stars, and providing valuable tools for scientific research and applications in medicine and industry. The study of radioactive decay provides invaluable insights into the structure of matter and the fundamental forces governing the universe.
Applications of Nuclear Physics: From Medicine to Energy
The study of the particles in the atomic nucleus has led to numerous groundbreaking applications across diverse fields. Nuclear medicine utilizes radioactive isotopes for diagnosis and treatment of diseases, providing powerful tools for imaging and targeted therapy. Nuclear power plants harness the energy released from nuclear fission to generate electricity. Nuclear physics also plays a vital role in various other fields, including materials science, archaeology, and geology.
Nuclear Medicine:
- Medical Imaging: Techniques like PET (positron emission tomography) and SPECT (single-photon emission computed tomography) use radioactive tracers to visualize internal organs and detect abnormalities.
- Radiation Therapy: Radioactive isotopes and particle beams are employed in cancer treatment to target and destroy cancerous cells.
Nuclear Energy:
Nuclear power plants utilize nuclear fission, the splitting of heavy atomic nuclei, to release enormous amounts of energy. This energy is then converted into electricity, providing a significant source of power for many countries.
Conclusion: A Journey into the Heart of Matter
The particles found in the nucleus of an atom, protons and neutrons, are not simply inert components but rather complex entities with fundamental properties that govern the behavior of matter. Understanding their composition, interactions, and the forces that bind them is crucial for unlocking the secrets of the universe. The ongoing research in nuclear physics continues to unveil fascinating insights into the structure of matter and the fundamental forces that shape our reality. From the stability of atoms to the applications in medicine and energy production, the study of nuclear particles remains a cornerstone of modern science, pushing the boundaries of our understanding of the physical world.
Latest Posts
Latest Posts
-
What Is Atmospheric Pressure In Kpa
Mar 24, 2025
-
Notes Above And Below The Staff
Mar 24, 2025
-
Calculate The Number Of Molecules In 4 00 Moles H2s
Mar 24, 2025
-
How Is An Ion Different From An Atom
Mar 24, 2025
-
What Percentage Is 12 Out Of 15
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about The Particles Found In The Nucleus Of An Atom Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
