The Most Abundant Gas In The Atmosphere
listenit
Mar 27, 2025 · 6 min read
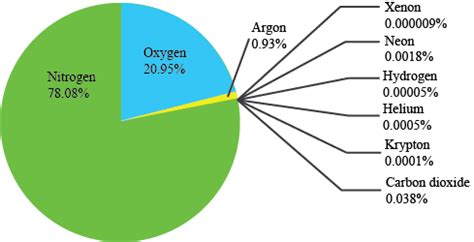
Table of Contents
The Most Abundant Gas in the Atmosphere: Nitrogen and Its Importance
Nitrogen, a colorless, odorless, and tasteless gas, reigns supreme as the most abundant component of Earth's atmosphere, making up a whopping 78% of its volume. Understanding its properties, role in various processes, and impact on life as we know it is crucial to grasping the complexities of our planet's systems. This comprehensive article delves deep into the world of nitrogen, exploring its atmospheric dominance, its various forms and cycles, its importance to life, and its anthropogenic impacts.
The Composition of Earth's Atmosphere: Nitrogen's Predominance
Earth's atmosphere is a dynamic mixture of various gases, each playing a unique role. While oxygen is essential for respiration, nitrogen's abundance significantly shapes the planet's environment and the life it sustains. The atmospheric composition is approximately:
- Nitrogen (N₂): 78%
- Oxygen (O₂): 21%
- Argon (Ar): 0.93%
- Carbon Dioxide (CO₂): 0.04%
- Other Gases (Neon, Helium, Methane, Krypton, Hydrogen, etc.): Trace amounts
This composition, especially the high nitrogen concentration, is not accidental. It's the result of billions of years of geological and biological processes. The significant abundance of nitrogen stems from its inert nature, its relative stability in the atmosphere, and its continuous recycling through the nitrogen cycle.
The Inert Nature of Atmospheric Nitrogen
Unlike oxygen, which readily participates in chemical reactions, atmospheric nitrogen (N₂) exists as a diatomic molecule with a strong triple bond. This triple bond requires substantial energy to break, making it relatively unreactive under normal atmospheric conditions. This inertness prevents rapid depletion of atmospheric nitrogen and contributes to its sustained high concentration.
The Nitrogen Cycle: A Continuous Process of Transformation
Despite its inertness, nitrogen is far from static. It participates in a vital biogeochemical cycle known as the nitrogen cycle. This cycle involves a series of transformations that convert nitrogen from one form to another, making it available to living organisms. The key processes are:
1. Nitrogen Fixation: Converting Atmospheric Nitrogen into Usable Forms
Atmospheric nitrogen, in its diatomic form (N₂), is largely inaccessible to most organisms. Nitrogen fixation is the crucial process by which atmospheric nitrogen is converted into ammonia (NH₃) or nitrate (NO₃⁻), forms that can be utilized by plants and other organisms. This transformation is primarily achieved through:
-
Biological Nitrogen Fixation: Certain bacteria, known as diazotrophs, possess the remarkable ability to break the strong triple bond in N₂ and convert it into ammonia. These bacteria live freely in the soil or form symbiotic relationships with plants, particularly legumes (peas, beans, etc.). The enzyme nitrogenase, found in these bacteria, plays a central role in this process.
-
Industrial Nitrogen Fixation (Haber-Bosch Process): This human-made process, invented in the early 20th century, converts atmospheric nitrogen into ammonia under high pressure and temperature using a catalyst. This ammonia is primarily used to produce fertilizers, significantly impacting global food production.
2. Nitrification: Converting Ammonia to Nitrates
Once ammonia is produced, it undergoes nitrification, a process where specialized bacteria convert it into nitrites (NO₂⁻) and then into nitrates (NO₃⁻). Nitrates are the primary form of nitrogen that plants absorb through their roots.
3. Assimilation: Plants and Animals Utilizing Nitrogen
Plants absorb nitrates from the soil and incorporate them into their tissues, forming essential organic nitrogen compounds like amino acids, proteins, and nucleic acids. Animals obtain nitrogen by consuming plants or other animals, thus incorporating nitrogen into their own biological structures.
4. Ammonification: Decomposition and Release of Ammonia
When plants and animals die, decomposers (bacteria and fungi) break down their organic matter, releasing nitrogen back into the environment in the form of ammonia.
5. Denitrification: Returning Nitrogen to the Atmosphere
Denitrifying bacteria convert nitrates back into gaseous nitrogen (N₂), which is then released back into the atmosphere, completing the cycle. This process occurs under anaerobic (oxygen-deficient) conditions.
The Importance of Nitrogen to Life
Nitrogen is an essential element for all life forms. It is a fundamental component of:
- Amino Acids: The building blocks of proteins, crucial for structural support, enzyme function, and numerous other biological processes.
- Nucleic Acids (DNA and RNA): The molecules that carry genetic information and direct protein synthesis.
- Chlorophyll: The pigment essential for photosynthesis in plants.
Without a sufficient supply of nitrogen, plant growth would be severely limited, impacting the entire food chain. The nitrogen cycle is therefore a cornerstone of ecosystem productivity and the stability of global food systems.
Anthropogenic Impacts on the Nitrogen Cycle
Human activities have significantly altered the natural nitrogen cycle, leading to both positive and negative consequences:
1. Increased Nitrogen Fixation through Fertilizer Production:
The Haber-Bosch process has dramatically increased the amount of nitrogen fixed globally. While this has boosted food production, it has also led to excess nitrogen in the environment.
2. Eutrophication: Excessive Nitrogen in Water Bodies
Excess nitrogen from fertilizers and other sources can run off into water bodies, causing eutrophication. This process leads to algal blooms, oxygen depletion, and the death of aquatic life. "Dead zones" in coastal waters are a significant consequence of this phenomenon.
3. Greenhouse Gas Emissions: Nitrous Oxide
Nitrous oxide (N₂O), a potent greenhouse gas, is released during denitrification and through certain agricultural practices. Its contribution to climate change is becoming increasingly concerning.
4. Acid Rain: Nitrogen Oxides
Nitrogen oxides (NOx) produced during combustion processes contribute to acid rain, which damages forests, aquatic ecosystems, and infrastructure.
5. Air Pollution: Nitrogen Dioxide
Nitrogen dioxide (NO₂) is a major air pollutant that contributes to respiratory problems and other health issues. It's a significant component of smog in urban areas.
Mitigating Anthropogenic Impacts on the Nitrogen Cycle
Addressing the negative impacts of human activities on the nitrogen cycle requires a multi-pronged approach:
- Improving Fertilizer Management: Implementing efficient fertilizer application techniques to minimize runoff and leaching.
- Developing Alternative Nitrogen Sources: Exploring alternative sources of nitrogen for agriculture, such as organic farming practices and biofertilizers.
- Reducing Fossil Fuel Consumption: Lowering emissions of nitrogen oxides from combustion sources.
- Protecting and Restoring Wetlands: Wetlands play a crucial role in nitrogen cycling and can help mitigate excess nitrogen in the environment.
- Promoting Sustainable Agriculture Practices: Implementing practices that minimize nitrogen losses and promote soil health.
Conclusion: The Vital Role of Nitrogen in a Changing World
Nitrogen, despite its inert nature, plays a pivotal role in shaping our planet and sustaining life. Its atmospheric dominance, its crucial involvement in the nitrogen cycle, and its importance as a fundamental building block of life highlight its indispensable significance. However, human activities have significantly altered the natural nitrogen cycle, leading to various environmental challenges. Addressing these challenges through sustainable practices and innovative technologies is crucial for maintaining a healthy planet and ensuring food security for future generations. The ongoing research and development in nitrogen management techniques are essential to navigate this complex interplay between human needs and environmental sustainability. Understanding the intricacies of the nitrogen cycle and its anthropogenic impacts is therefore not only scientifically important but also crucial for informed decision-making in addressing global environmental issues. The future of our planet hinges on a sustainable relationship with this most abundant gas in our atmosphere.
Latest Posts
Latest Posts
-
24 Is What Percent Of 25
Mar 30, 2025
-
Basic Unit Of Structure And Function In All Living Things
Mar 30, 2025
-
2x Y 4 In Slope Intercept Form
Mar 30, 2025
-
What State Of Matter Has The Most Kinetic Energy
Mar 30, 2025
-
What Is The Conflict In The Book The Giver
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about The Most Abundant Gas In The Atmosphere . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
