The Final Electron Acceptor In The Electron Transport Chain Is
listenit
Apr 01, 2025 · 5 min read
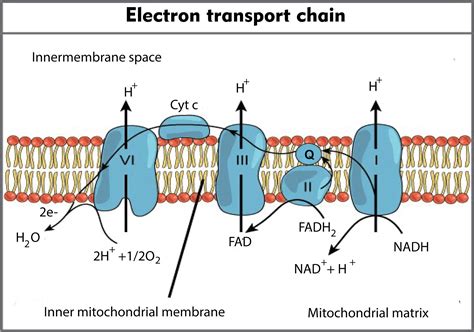
Table of Contents
The Final Electron Acceptor in the Electron Transport Chain Is... Oxygen! Understanding its Crucial Role in Cellular Respiration
The electron transport chain (ETC), a vital component of cellular respiration, is a complex series of protein complexes embedded within the inner mitochondrial membrane. Its primary function is to harness the energy stored in electrons derived from the breakdown of glucose and other nutrients, ultimately converting it into a usable form of energy – ATP (adenosine triphosphate). This process relies heavily on a series of redox reactions, where electrons are passed from one molecule to another, gradually decreasing in energy level. But the entire system hinges on a crucial component: the final electron acceptor. This role is fulfilled by oxygen (O2).
The Electron Transport Chain: A Cascade of Redox Reactions
Before delving into the importance of oxygen, let's briefly review the ETC itself. The chain consists of four major protein complexes (Complexes I-IV), along with two mobile electron carriers – ubiquinone (CoQ) and cytochrome c. Each complex contains metal ions, particularly iron-sulfur clusters and cytochromes, which facilitate the transfer of electrons.
The process begins with Complex I (NADH dehydrogenase), where electrons from NADH (nicotinamide adenine dinucleotide), a high-energy electron carrier produced during glycolysis and the Krebs cycle, are transferred. These electrons then move down the chain through CoQ to Complex III (cytochrome bc1 complex) and subsequently to cytochrome c. Finally, the electrons reach Complex IV (cytochrome c oxidase).
At each step, the electrons lose energy, which is harnessed to pump protons (H+) from the mitochondrial matrix across the inner mitochondrial membrane into the intermembrane space. This creates a proton gradient, a difference in proton concentration across the membrane, which is crucial for ATP synthesis. This proton gradient drives ATP production through a process called chemiosmosis, where protons flow back into the matrix through ATP synthase, an enzyme that uses the energy of this flow to produce ATP.
Oxygen: The Terminal Electron Acceptor and its Indispensable Role
The entire ETC process wouldn't be possible without a final electron acceptor to receive the electrons at the end of the chain. This is where oxygen comes into play. Oxygen is the terminal electron acceptor because it has a high electronegativity; it strongly attracts electrons.
How Oxygen Accepts Electrons:
In Complex IV, four electrons are ultimately transferred to one oxygen molecule (O2). Simultaneously, four protons from the matrix are consumed in the reaction. This process forms two molecules of water (H2O), a byproduct of cellular respiration. The reaction can be summarized as:
4e⁻ + 4H⁺ + O₂ → 2H₂O
Without oxygen, the electron transport chain would come to a halt. The electrons would accumulate in the chain, preventing further electron transfer and thus halting the proton pumping. Consequently, the proton gradient would not be established, and ATP synthesis would cease. This is the reason why oxygen is essential for aerobic respiration.
The Consequences of Oxygen Absence: Anaerobic Respiration and Fermentation
In the absence of oxygen, organisms resort to alternative mechanisms for energy production. These include anaerobic respiration and fermentation.
Anaerobic Respiration: Some organisms can utilize alternative electron acceptors such as nitrate (NO3⁻), sulfate (SO4²⁻), or even carbon dioxide (CO2). However, these alternative acceptors are less efficient than oxygen, leading to less ATP production.
Fermentation: This is a less efficient process that doesn't involve the electron transport chain. Instead, it regenerates NAD+ from NADH, allowing glycolysis to continue producing a small amount of ATP in the absence of oxygen. Examples of fermentation include lactic acid fermentation (in muscles during strenuous exercise) and alcoholic fermentation (in yeast).
The Significance of Oxygen's Role: Beyond ATP Production
Oxygen's role extends beyond simply acting as the final electron acceptor in the ETC. It plays a crucial role in several other aspects of cellular metabolism and overall health:
-
Reactive Oxygen Species (ROS) Production and Antioxidant Defense: While oxygen is essential, its reduction in Complex IV can lead to the formation of reactive oxygen species (ROS), such as superoxide radicals (O₂⁻) and hydrogen peroxide (H₂O₂). ROS are highly reactive molecules that can damage cellular components like DNA, proteins, and lipids, contributing to aging and various diseases. To combat this, cells have evolved intricate antioxidant defense systems that neutralize ROS.
-
Regulation of Gene Expression: Oxygen levels can influence gene expression, affecting various cellular processes. For example, low oxygen levels (hypoxia) trigger the expression of genes involved in angiogenesis (formation of new blood vessels) and erythropoiesis (red blood cell production).
-
Oxygen Sensing and Homeostasis: The body has sophisticated mechanisms to sense oxygen levels and maintain oxygen homeostasis. Specialized cells, such as those in the carotid bodies, detect changes in oxygen tension and send signals to the respiratory centers in the brain, adjusting breathing rate to maintain adequate oxygen supply.
Oxygen Deficiency and its Health Implications
Oxygen deficiency, or hypoxia, can have severe consequences for the body. Prolonged hypoxia can lead to various health problems, including:
-
Tissue Damage: Without sufficient oxygen, cells cannot produce enough ATP, leading to impaired cellular function and eventual cell death. This can damage tissues and organs, potentially causing organ failure.
-
Cardiovascular Problems: Hypoxia can impair heart function, leading to reduced blood flow and potentially heart failure.
-
Neurological Disorders: The brain is highly sensitive to oxygen deprivation. Hypoxia can cause brain damage, leading to neurological disorders, including seizures and coma.
-
Respiratory Diseases: Conditions that limit oxygen uptake, such as chronic obstructive pulmonary disease (COPD) and pneumonia, lead to hypoxia and its associated complications.
Understanding Oxygen's Role: A Key to Health and Disease
The final electron acceptor in the electron transport chain, oxygen, plays a pivotal role in cellular respiration and overall human health. Its role is not just about ATP production; it also influences ROS production, gene expression, and overall cellular homeostasis. Understanding the intricate mechanisms involved in oxygen utilization and the consequences of oxygen deficiency is crucial for comprehending various physiological processes and developing strategies for preventing and treating diseases associated with impaired oxygen metabolism. Further research continues to illuminate the complexities of oxygen's multifaceted roles, offering valuable insights into human health and disease. The importance of maintaining adequate oxygen levels through proper nutrition, exercise, and avoidance of environmental toxins cannot be overstated.
Latest Posts
Latest Posts
-
What Is The Limit Of A Constant
Apr 02, 2025
-
X 3 2x 2 X 2 0
Apr 02, 2025
-
1 3 Divided By 1 6 As A Fraction
Apr 02, 2025
-
How To Determine Zeros Of A Function
Apr 02, 2025
-
What Are Four Principles Of Natural Selection
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The Final Electron Acceptor In The Electron Transport Chain Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
