The Coefficient In A Chemical Equation Represents
listenit
Mar 26, 2025 · 5 min read
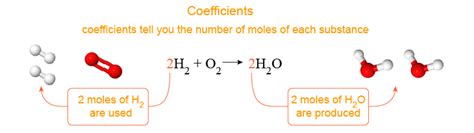
Table of Contents
The Coefficient in a Chemical Equation Represents: A Deep Dive into Stoichiometry
Understanding chemical reactions is fundamental to chemistry. A balanced chemical equation provides a concise representation of the reaction, showing the reactants transforming into products. Central to interpreting these equations are the coefficients – numbers placed in front of chemical formulas. But what exactly do these coefficients represent? This article will delve deep into the meaning and significance of coefficients in chemical equations, exploring their implications for stoichiometry, calculations, and understanding chemical processes at a fundamental level.
What Coefficients Represent: The Foundation of Stoichiometry
The coefficient in a chemical equation represents the relative number of moles of each reactant and product involved in the reaction. It's crucial to understand that it's not just about the number of molecules or atoms, but the moles. One mole is a specific quantity, containing Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of particles (atoms, molecules, ions, etc.). This is essential for bridging the microscopic world of atoms and molecules to the macroscopic world of laboratory measurements.
Example:
Consider the combustion of methane:
CH<sub>4</sub> + 2O<sub>2</sub> → CO<sub>2</sub> + 2H<sub>2</sub>O
- Coefficient of CH<sub>4</sub> is 1: This means one mole of methane reacts.
- Coefficient of O<sub>2</sub> is 2: This indicates two moles of oxygen gas are needed for the reaction.
- Coefficient of CO<sub>2</sub> is 1: One mole of carbon dioxide is produced.
- Coefficient of H<sub>2</sub>O is 2: Two moles of water are formed.
These coefficients maintain the law of conservation of mass, ensuring that the number of atoms of each element remains the same on both sides of the equation. In this example, we have one carbon atom, four hydrogen atoms, and four oxygen atoms on both the reactant and product sides.
Beyond Mole Ratios: The Implications of Coefficients
The significance of coefficients extends beyond simply expressing mole ratios. They are the cornerstone of stoichiometric calculations, allowing us to determine:
1. Mass Relationships:
Coefficients provide the link between the moles of reactants and products and their respective masses. Using molar mass (grams per mole) for each substance, we can convert the mole ratios into mass ratios. This is crucial for determining the amount of product formed from a given amount of reactant or the amount of reactant needed to produce a desired amount of product.
Example: To calculate the mass of CO<sub>2</sub> produced from 16 grams of CH<sub>4</sub>, we'd use the coefficients to determine the mole ratio and then molar masses to convert moles to grams.
2. Volume Relationships (for Gases):
For reactions involving gases at the same temperature and pressure, the coefficients also represent the volume ratios. This is a direct consequence of Avogadro's law, which states that equal volumes of gases at the same temperature and pressure contain the same number of molecules.
Example: In the methane combustion reaction, the coefficients indicate that one volume of methane reacts with two volumes of oxygen to produce one volume of carbon dioxide and two volumes of water vapor (assuming all are gases at the same T and P).
3. Limiting Reactants and Percent Yield:
In real-world reactions, reactants are often not present in the exact stoichiometric ratios indicated by the coefficients. One reactant will be completely consumed before others, becoming the limiting reactant. The coefficients are crucial for identifying the limiting reactant and calculating the theoretical yield (maximum amount of product possible). Comparing the actual yield (amount of product actually obtained) to the theoretical yield gives the percent yield, a measure of reaction efficiency.
Example: If we have less than two moles of O<sub>2</sub> for every mole of CH<sub>4</sub>, oxygen will be the limiting reactant, and the amount of CO<sub>2</sub> formed will be limited by the available oxygen.
4. Understanding Reaction Mechanisms:
While coefficients don't directly reveal the reaction mechanism (the step-by-step process of a reaction), they can offer clues. For example, a reaction with a large coefficient for an intermediate species might suggest a complex mechanism involving multiple steps. However, interpreting reaction mechanisms requires more detailed kinetic studies.
Beyond the Basics: Advanced Applications of Coefficients
The importance of coefficients in chemical equations extends to more advanced areas of chemistry:
1. Equilibrium Calculations:
In reversible reactions, the coefficients are incorporated into the equilibrium constant expression (K<sub>c</sub> or K<sub>p</sub>), which quantifies the relative amounts of reactants and products at equilibrium. The coefficients appear as exponents in the equilibrium constant expression.
2. Thermodynamics:
Coefficients play a role in thermodynamic calculations, such as determining the standard enthalpy change (ΔH°) or standard entropy change (ΔS°) for a reaction. These changes are often expressed per mole of reaction, meaning the coefficients dictate the scaling of the energy changes involved.
3. Electrochemistry:
In electrochemical reactions, the coefficients are crucial for determining the number of electrons transferred in a redox reaction. This is essential for calculating the cell potential (voltage) using the Nernst equation.
Common Mistakes and Misconceptions
Several common misconceptions surround coefficients:
- Coefficients are not subscripts: Coefficients represent the number of molecules or moles while subscripts indicate the number of atoms within a molecule. Confusing these leads to incorrect calculations and interpretations.
- Coefficients cannot be changed arbitrarily: Balancing a chemical equation involves adjusting coefficients to satisfy the law of conservation of mass. Arbitrarily altering coefficients renders the equation unbalanced and inaccurate.
- Coefficients don't always represent the relative number of molecules directly: While often representing the simple ratio, this can be misleading for reactions involving complex mechanisms or large molecules.
Conclusion: The Power of Coefficients in Chemical Equations
The coefficients in a chemical equation are not mere numbers; they are the foundation of stoichiometry and hold immense significance in interpreting and manipulating chemical reactions. They provide the quantitative relationships between reactants and products, enabling calculations of mass, volume, limiting reactants, percent yield, equilibrium constants, and thermodynamic properties. A thorough understanding of coefficients is essential for anyone working in chemistry, from introductory students to advanced researchers. By grasping their meaning and applications, we can unlock a deeper comprehension of the quantitative nature of chemical transformations and the elegance of balanced chemical equations. Mastering this concept empowers a more profound understanding of the chemical world around us.
Latest Posts
Latest Posts
-
How Many Light Years Away Is Mars From Earth
Mar 29, 2025
-
Whats The Square Root Of 23
Mar 29, 2025
-
What Is The Decimal For 6 10
Mar 29, 2025
-
What Is The Square Root Of 2000
Mar 29, 2025
-
What Are 4 Agents Of Erosion
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about The Coefficient In A Chemical Equation Represents . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
