The Center Of An Atom Is Called The
listenit
Mar 26, 2025 · 6 min read
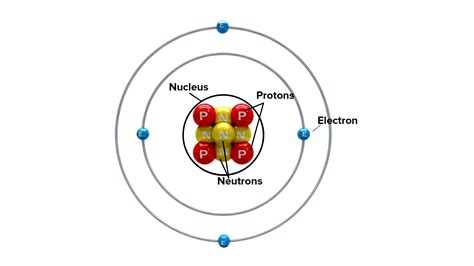
Table of Contents
The Center of an Atom is Called the Nucleus: A Deep Dive into Atomic Structure
The atom, the fundamental building block of matter, is a fascinating and complex entity. At its heart lies the nucleus, a tiny but incredibly dense region containing the majority of the atom's mass and all of its positive charge. Understanding the nucleus is key to understanding the behavior of atoms, molecules, and ultimately, all the matter around us. This article will delve deep into the nucleus, exploring its composition, properties, and importance in various scientific fields.
What is the Atomic Nucleus?
The atomic nucleus is the positively charged central core of an atom, composed of protons and neutrons. These subatomic particles are collectively known as nucleons. The nucleus is incredibly small compared to the overall size of the atom; if an atom were the size of a football stadium, the nucleus would be about the size of a pea in the center. Despite its minuscule size, the nucleus holds the vast majority of the atom's mass, contributing over 99.9% of its total weight.
Protons: The Positive Charge Carriers
Protons are positively charged particles with a mass approximately 1836 times greater than that of an electron. The number of protons in an atom's nucleus, known as the atomic number, determines the atom's identity and its position on the periodic table. All atoms of a given element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six protons, and so on.
Neutrons: The Neutral Partners
Neutrons, as their name suggests, carry no electrical charge. Their mass is very slightly larger than that of a proton. Neutrons play a crucial role in stabilizing the nucleus. The number of neutrons in an atom can vary, even within the same element, leading to different isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. Some isotopes are stable, while others are radioactive, undergoing decay to become more stable.
The Strong Nuclear Force: Holding the Nucleus Together
The protons within the nucleus are all positively charged, and like charges repel each other. This repulsive force should cause the nucleus to fly apart. However, the nucleus remains stable due to the strong nuclear force, one of the four fundamental forces in nature. This force is much stronger than the electromagnetic force of repulsion between protons, but it acts only over very short distances—within the nucleus itself. The strong nuclear force is responsible for binding the protons and neutrons together, overcoming the electromagnetic repulsion and maintaining the integrity of the nucleus.
Isotopes and Radioactive Decay
As mentioned, isotopes are atoms of the same element with varying numbers of neutrons. Some isotopes are stable, meaning their nuclei remain intact. However, many isotopes are unstable or radioactive, meaning their nuclei spontaneously decay over time, emitting particles or energy in the process. This decay can transform the atom into a different element. Radioactive decay is a fundamental process in nuclear physics with applications in various fields, including medicine, archaeology, and geological dating.
Nuclear Fission and Fusion
The nucleus is also the site of nuclear reactions, which involve changes in the composition of the nucleus. Nuclear fission is the splitting of a heavy nucleus into two or more lighter nuclei, releasing a tremendous amount of energy. This process is used in nuclear power plants and nuclear weapons. Nuclear fusion, on the other hand, involves the combining of two light nuclei into a heavier nucleus, also releasing vast amounts of energy. This is the process that powers the sun and other stars.
The Nucleus and Chemical Properties
While the nucleus determines the element's identity, it's primarily the electrons orbiting the nucleus that determine the atom's chemical properties. The arrangement of electrons in energy levels and orbitals influences how an atom interacts with other atoms, forming chemical bonds and participating in chemical reactions. Although the nucleus is largely unaffected by chemical reactions, its properties, particularly the number of protons, indirectly influence the atom's chemical behavior through its effect on the electron configuration.
Investigating the Nucleus: Techniques and Technologies
Studying the nucleus requires advanced techniques and technologies due to its incredibly small size and the strong forces involved. Several methods are employed:
Particle Accelerators
Particle accelerators, like cyclotrons and synchrotrons, are used to accelerate charged particles to high energies, enabling scientists to probe the structure of the nucleus by colliding these particles with atomic nuclei. These collisions can reveal information about the constituents of the nucleus and the forces governing their interactions.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy utilizes the magnetic properties of atomic nuclei to study molecular structure and dynamics. By applying a magnetic field and radio waves, scientists can obtain detailed information about the arrangement and interactions of atoms within molecules, providing valuable insights into chemical processes.
Mass Spectrometry
Mass spectrometry is a technique used to determine the mass-to-charge ratio of ions. This allows scientists to identify different isotopes of an element and measure their relative abundances, providing information about the nuclear composition of a sample.
The Nucleus and its Significance
The nucleus's importance extends far beyond the realm of basic atomic structure. Its significance is evident in several areas:
Nuclear Medicine
Radioactive isotopes are widely used in nuclear medicine for diagnosis and treatment. Techniques like Positron Emission Tomography (PET) scans utilize radioactive tracers to image metabolic processes within the body, aiding in the detection and diagnosis of various diseases. Radioactive isotopes are also employed in radiation therapy for cancer treatment.
Nuclear Power Generation
Nuclear fission is employed in nuclear power plants to generate electricity. The controlled fission of uranium or plutonium nuclei releases vast amounts of energy, which is harnessed to produce steam and drive turbines, generating electricity. This provides a significant source of power in many countries.
Radiocarbon Dating
Radiocarbon dating, utilizing the radioactive decay of carbon-14, is a powerful tool in archaeology and paleontology for determining the age of organic materials. By measuring the remaining amount of carbon-14 in a sample, scientists can estimate its age, providing valuable insights into past civilizations and ecosystems.
Geological Dating
Similar to radiocarbon dating, other radioactive isotopes are used to date geological formations and rocks. This allows scientists to reconstruct the Earth's history, understand geological processes, and determine the age of various minerals and formations.
The Ongoing Mysteries of the Nucleus
Despite significant advances in our understanding of the nucleus, many mysteries remain. Researchers continue to explore fundamental questions about nuclear structure, interactions, and behavior. Areas of ongoing research include:
- The nature of the strong nuclear force: While we understand its effects, a complete theoretical understanding of the strong nuclear force remains a challenge.
- The structure of exotic nuclei: Scientists are studying nuclei far from the stability line, which exhibit unusual properties and decay modes.
- The search for new fundamental particles: Researchers continue to search for evidence of new particles and interactions that could provide insights into the fundamental laws of physics governing nuclear processes.
The nucleus, the atom's core, is a microcosm of the universe, holding a wealth of scientific significance and prompting continuous research. Its study provides crucial insights into the fundamental laws of physics, offers practical applications in numerous fields, and continues to unveil fascinating mysteries about the nature of matter itself. From its role in powering stars to its application in medical diagnosis and treatment, the nucleus remains a central subject of scientific inquiry and technological advancement. Understanding its intricacies is essential for pushing the boundaries of knowledge and harnessing the power of the atom for the benefit of humanity.
Latest Posts
Latest Posts
-
What Is 6 To The Power Of 4
Mar 27, 2025
-
What Is 1 3 Divided By 6
Mar 27, 2025
-
How Many Chromosomes Do Mice Have
Mar 27, 2025
-
In What Units Is Frequency Measured
Mar 27, 2025
-
Whats The Lcm Of 6 And 10
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about The Center Of An Atom Is Called The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
