Strong Base Titrated With Weak Acid
listenit
Mar 13, 2025 · 6 min read
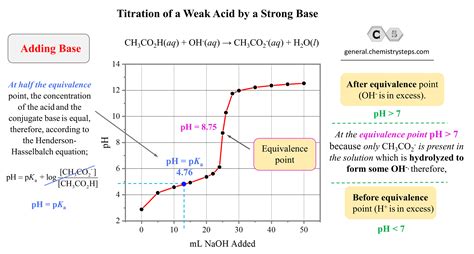
Table of Contents
Strong Base Titrated with Weak Acid: A Comprehensive Guide
Understanding acid-base titrations is crucial in chemistry, offering a practical method for determining the concentration of an unknown solution. This article delves into the specifics of titrating a strong base with a weak acid, exploring the underlying chemistry, calculations, and practical applications. We will cover the key concepts in detail, offering a comprehensive guide suitable for students and professionals alike.
The Chemistry Behind the Titration
A titration involves the gradual addition of a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte) until the reaction is complete. In the case of a strong base titrated with a weak acid, the strong base, typically sodium hydroxide (NaOH) or potassium hydroxide (KOH), completely dissociates in solution, yielding a high concentration of hydroxide ions (OH⁻). The weak acid, on the other hand, only partially dissociates, resulting in a lower concentration of hydronium ions (H₃O⁺) and its conjugate base.
The reaction between the strong base and weak acid is a neutralization reaction:
OH⁻(aq) + HA(aq) ⇌ A⁻(aq) + H₂O(l)
Where:
- OH⁻(aq) represents the hydroxide ions from the strong base.
- HA(aq) represents the weak acid.
- A⁻(aq) represents the conjugate base of the weak acid.
- H₂O(l) represents water.
The equilibrium of this reaction lies far to the right, meaning the reaction proceeds almost completely to completion. However, the presence of the conjugate base, A⁻, significantly influences the pH of the solution throughout the titration.
Stages of the Titration
The titration curve, a graph of pH versus volume of titrant added, provides valuable insights into the titration process. Several distinct stages characterize the titration of a strong base with a weak acid:
-
Initial Stage (Before Titration): The pH of the strong base solution is initially high, reflecting the high concentration of hydroxide ions.
-
Buffer Region: As the weak acid is added, it reacts with the hydroxide ions, forming the conjugate base. This region creates a buffer solution, a solution that resists changes in pH. The pH changes relatively slowly in this region, due to the buffer's ability to neutralize added acid or base. The Henderson-Hasselbalch equation is crucial here for calculating the pH:
pH = pKa + log([A⁻]/[HA])
Where:
-
pKa is the negative logarithm of the acid dissociation constant (Ka) of the weak acid.
-
[A⁻] is the concentration of the conjugate base.
-
[HA] is the concentration of the weak acid.
-
Half-Equivalence Point: At the half-equivalence point, exactly half of the weak acid has been neutralized. At this point, the concentrations of the weak acid and its conjugate base are equal, simplifying the Henderson-Hasselbalch equation to:
pH = pKa
This makes the half-equivalence point a useful way to determine the pKa of the weak acid.
-
Equivalence Point: The equivalence point is reached when the moles of strong base added equal the moles of weak acid initially present. At this point, the solution contains only the conjugate base of the weak acid. The pH at the equivalence point is greater than 7, because the conjugate base of a weak acid is basic.
-
Post-Equivalence Point: After the equivalence point, the addition of further strong base results in a rapid increase in pH. The solution becomes increasingly basic, dominated by the excess hydroxide ions from the strong base.
Calculations and Data Analysis
Accurate calculations are vital for determining the concentration of the unknown strong base. The following steps outline a typical calculation process:
- Molarity Calculation: The concentration of the strong base can be calculated using the following equation:
M₁V₁ = M₂V₂
Where:
- M₁ is the molarity of the weak acid (known).
- V₁ is the volume of the weak acid (known).
- M₂ is the molarity of the strong base (unknown).
- V₂ is the volume of strong base at the equivalence point (determined experimentally).
-
Equivalence Point Determination: The equivalence point is typically determined from the titration curve by observing the point of steepest slope. Various methods, including the first derivative and second derivative methods, can be employed to accurately identify the equivalence point.
-
pH Calculation at Different Stages: Calculating the pH at different stages of the titration requires consideration of the different species present in the solution. The Henderson-Hasselbalch equation is especially useful in the buffer region. In the post-equivalence point, the excess hydroxide ion concentration determines the pH.
-
Error Analysis: It's crucial to consider sources of error, such as inaccurate measurements of volume, impurities in the solutions, and improper use of indicators. Proper error analysis ensures the reliability of the results.
Titration Curves and Indicators
The titration curve visually represents the change in pH during the titration. It's characterized by a gradual change in pH in the buffer region, followed by a sharp increase in pH near the equivalence point. The shape of the curve is influenced by the strength of the acid and base.
Choosing an appropriate indicator is essential for visually identifying the equivalence point. The indicator's pKa should be close to the pH at the equivalence point. Phenolphthalein, with a pKa of around 9.4, is often a suitable indicator for the titration of a strong base with a weak acid, because the equivalence point pH will be greater than 7. The color change of the indicator signals the completion of the neutralization reaction.
Practical Applications
The titration of a strong base with a weak acid finds application in various fields:
-
Environmental Monitoring: Determining the concentration of weak acids in environmental samples, such as wastewater or soil samples.
-
Food and Beverage Industry: Analyzing the acidity of food products.
-
Pharmaceutical Industry: Quality control of pharmaceutical products.
-
Medical Diagnostics: Analyzing body fluids for acid-base imbalances.
Advanced Considerations
-
Ionic Strength: The ionic strength of the solution can affect the activity coefficients of the ions and thus the pH of the solution.
-
Temperature Effects: Temperature changes can affect the equilibrium constant of the reaction and the solubility of the reactants.
-
Non-ideal Behavior: At high concentrations, deviations from ideal behavior can occur, necessitating corrections in the calculations.
Conclusion
The titration of a strong base with a weak acid is a fundamental concept in chemistry with far-reaching applications. Understanding the underlying chemistry, mastering the calculation techniques, and carefully interpreting the titration curve are essential for obtaining accurate and reliable results. This detailed guide provides a solid foundation for those seeking a deeper understanding of this important analytical technique. The principles discussed here extend beyond simple laboratory exercises, highlighting the vital role of acid-base titrations in various scientific and industrial fields. Careful attention to detail in experimental design and data analysis is crucial for harnessing the power of this technique. By understanding and applying these principles, students and professionals alike can effectively use strong base-weak acid titrations to accurately determine concentrations and solve real-world problems. Further exploration of related topics, such as potentiometric titrations and the use of advanced statistical methods in data analysis, will enhance one’s proficiency in this essential area of chemistry.
Latest Posts
Latest Posts
-
How Many Valence Electrons Are In Argon
Mar 13, 2025
-
How Is Genetic Drift Different From Natural Selection
Mar 13, 2025
-
A Repair Bill For Your Car Is 553
Mar 13, 2025
-
How Many Meters Is 100 Cm
Mar 13, 2025
-
Some Isosceles Triangles Are Not Equilateral
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Strong Base Titrated With Weak Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
