How Many Valence Electrons Are In Argon
listenit
Mar 13, 2025 · 5 min read
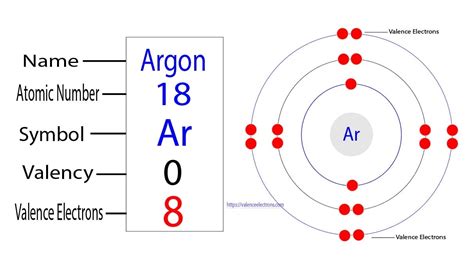
Table of Contents
How Many Valence Electrons Are in Argon? A Deep Dive into Atomic Structure
Argon, a noble gas residing serenely in Group 18 of the periodic table, holds a unique position in chemistry due to its exceptional stability. This stability is directly linked to its electron configuration and, crucially, the number of valence electrons it possesses. Understanding argon's valence electrons is key to understanding its chemical behavior and its significance in various applications. This article delves into the specifics of argon's electronic structure, explains the concept of valence electrons, and explores the implications of argon's full valence shell.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before we delve into the specifics of argon, let's establish a firm grasp on the concept of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. These electrons are the primary participants in chemical bonding, determining an atom's reactivity and the types of bonds it can form. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas, through gaining, losing, or sharing valence electrons.
The Significance of the Octet Rule: For many elements, particularly those in the main group (groups 1-18), the most stable electron configuration is achieved when the valence shell contains eight electrons – this is known as the octet rule. This rule, while not universally applicable, provides a valuable framework for understanding chemical bonding and reactivity. Noble gases, with their full valence shells, serve as the benchmark of stability.
Argon's Electron Configuration: A Stable Eight
Argon (Ar), with an atomic number of 18, possesses 18 electrons. These electrons are distributed across three energy levels (or shells) according to the Aufbau principle and Hund's rule. The electron configuration of argon is written as 1s²2s²2p⁶3s²3p⁶.
Let's break down this notation:
- 1s²: Two electrons occupy the first energy level (n=1) in the s orbital.
- 2s²: Two electrons occupy the second energy level (n=2) in the s orbital.
- 2p⁶: Six electrons occupy the second energy level (n=2) in the three p orbitals.
- 3s²: Two electrons occupy the third energy level (n=3) in the s orbital.
- 3p⁶: Six electrons occupy the third energy level (n=3) in the three p orbitals.
Determining Argon's Valence Electrons: Identifying the Outermost Shell
To determine the number of valence electrons in argon, we focus on the outermost electron shell, which in argon's case is the third energy level (n=3). This shell contains both the 3s and 3p orbitals, encompassing a total of 2 + 6 = 8 electrons.
Therefore, argon has eight valence electrons. This complete octet is the reason for argon's exceptional stability and its inert nature – it has no tendency to gain, lose, or share electrons to achieve a more stable configuration.
Argon's Inertness: A Consequence of its Full Valence Shell
The presence of eight valence electrons in argon directly accounts for its inertness – its unwillingness to participate in chemical reactions. This characteristic is shared by all other noble gases. Their full valence shells make them extremely stable and resistant to forming chemical bonds. This stability is a fundamental aspect of their chemical properties.
Implications of Argon's Inertness: Argon's lack of reactivity makes it exceptionally useful in various applications where inertness is crucial. For instance:
- Welding: Argon is used as a shielding gas in welding processes to prevent oxidation and contamination of the weld. Its inertness protects the molten metal from reacting with the surrounding atmosphere.
- Light Bulbs: Argon is used in incandescent light bulbs to extend the lifespan of the filament. Its inertness prevents the filament from reacting with oxygen, thus preventing premature burnout.
- Medical Applications: Argon is employed in some medical procedures as a cryogenic agent due to its ability to remain inert at low temperatures.
- Other Industrial Applications: Argon’s inertness renders it suitable for numerous industrial applications such as preventing oxidation in chemical processes and preserving samples in analytical chemistry.
Comparing Argon to Other Elements: A Look at Valence Electron Trends
Understanding argon's valence electrons allows for a comparison with other elements and a further appreciation of periodic trends. For example:
- Chlorine (Cl): Chlorine, located in Group 17, has seven valence electrons. This explains its high reactivity, as it readily gains one electron to achieve a stable octet, forming the chloride ion (Cl⁻).
- Potassium (K): Potassium, located in Group 1, has only one valence electron. It readily loses this electron to achieve a stable configuration, forming the potassium ion (K⁺).
- Other Noble Gases: All noble gases possess eight valence electrons (except helium, which has two), accounting for their shared inertness.
Beyond the Octet Rule: Exceptions and Advanced Concepts
While the octet rule provides a helpful framework, it is essential to acknowledge its limitations. Some elements, particularly those in the later periods of the periodic table, can exhibit expanded octets, meaning they can accommodate more than eight valence electrons. This is due to the involvement of d and f orbitals in bonding.
However, Argon, being a second-period element, strictly adheres to the octet rule, reinforcing its inertness and the stability associated with its eight valence electrons.
The Importance of Valence Electrons in Chemistry and Beyond
The concept of valence electrons is fundamental to understanding chemical behavior and predicting the properties of elements. Knowing the number of valence electrons allows chemists to:
- Predict chemical bonding: The number of valence electrons determines the types of bonds an atom will form (ionic, covalent, metallic).
- Understand reactivity: Atoms with incomplete valence shells tend to be more reactive than those with complete valence shells.
- Explain periodic trends: Many periodic trends, such as electronegativity and ionization energy, are directly related to the number of valence electrons.
Conclusion: Argon's Eight Valence Electrons – A Foundation of Stability
In conclusion, argon possesses eight valence electrons, a complete octet that directly explains its exceptional stability and inertness. This characteristic is pivotal in understanding its chemical behavior and its wide range of applications in various scientific and industrial fields. The study of valence electrons remains a cornerstone of chemistry, providing a framework for comprehending the interactions between atoms and the formation of molecules and compounds. The unique properties of argon, stemming from its full valence shell, highlight the importance of this fundamental concept in the world of chemistry and beyond. The implications extend far beyond simply knowing the number eight; it unveils the underlying principles of atomic structure and chemical reactivity.
Latest Posts
Latest Posts
-
What Is The Square Root Of 128
Mar 13, 2025
-
2 And 2 3 As An Improper Fraction
Mar 13, 2025
-
X 2y 4 In Slope Intercept Form
Mar 13, 2025
-
1 Sinx Cosx Cosx 1 Sinx
Mar 13, 2025
-
What Is The Difference Between Community And Population
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Argon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
