Sodium Hydroxide Is A Strong Base
listenit
Mar 31, 2025 · 6 min read
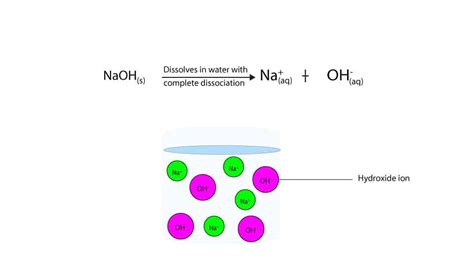
Table of Contents
Sodium Hydroxide: A Deep Dive into the Properties and Applications of a Strong Base
Sodium hydroxide (NaOH), also known as lye or caustic soda, is a highly alkaline inorganic compound. Its strong base nature makes it incredibly versatile, finding applications in diverse industries ranging from chemical manufacturing to food processing. Understanding its properties and applications is crucial for safe and effective use. This comprehensive guide delves into the chemical characteristics, reactions, safety precautions, and wide-ranging applications of sodium hydroxide.
Understanding the Strong Base Nature of Sodium Hydroxide
Sodium hydroxide's strength as a base stems from its complete dissociation in aqueous solutions. When NaOH is dissolved in water, it readily ionizes into sodium ions (Na⁺) and hydroxide ions (OH⁻). This complete ionization is the hallmark of a strong base, contrasting with weak bases that only partially ionize. The high concentration of hydroxide ions (OH⁻) is what gives sodium hydroxide its characteristic high pH, typically above 12. This high pH indicates a high concentration of hydroxide ions, signifying its strong basicity.
The Chemical Equation for Dissociation
The dissociation of sodium hydroxide in water is represented by the following chemical equation:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
This equation shows the complete breakdown of NaOH into its constituent ions, sodium cation (Na⁺) and hydroxide anion (OH⁻). There is no equilibrium indicated because the reaction proceeds essentially to completion in aqueous solution. This complete dissociation is key to understanding its strong base properties and its reactivity.
Key Properties of Sodium Hydroxide
Beyond its strong base nature, several other properties contribute to sodium hydroxide's versatility:
-
High Solubility: NaOH is highly soluble in water, readily dissolving to form concentrated alkaline solutions. This solubility is critical for its use in various industrial processes where it needs to be dissolved and used as a solution.
-
Hygroscopic Nature: Sodium hydroxide is hygroscopic, meaning it readily absorbs moisture from the air. This property necessitates careful storage to prevent clumping and degradation. Airtight containers are essential for preserving its purity and effectiveness.
-
Reactivity: Its strong base nature makes it highly reactive with various substances, including acids, metals, and organic compounds. These reactions often produce heat, making careful handling essential.
-
Appearance: Pure sodium hydroxide is a white, crystalline solid. However, commercially available forms might appear slightly off-white due to impurities.
Chemical Reactions of Sodium Hydroxide
Sodium hydroxide's strong basicity leads to a range of characteristic chemical reactions:
1. Neutralization Reactions with Acids
NaOH readily reacts with acids in neutralization reactions, producing salt and water. This is a fundamental chemical reaction used widely in titrations and acid-base chemistry. For example, the reaction with hydrochloric acid (HCl) is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This reaction releases heat (exothermic). The strength of the reaction depends on the concentration of the acid and the base.
2. Saponification Reactions
NaOH plays a crucial role in saponification, the process of making soap. It reacts with fats and oils (triglycerides) to produce glycerol and soap (fatty acid salts). This is a classic example of a base-catalyzed reaction where the hydroxide ions initiate the hydrolysis of ester bonds in triglycerides.
3. Reactions with Metals
NaOH reacts with certain metals, particularly amphoteric metals like aluminum and zinc, to produce hydrogen gas and metal salts. This reaction is highly exothermic and generates considerable heat. For example, the reaction with aluminum is:
2NaOH(aq) + 2Al(s) + 6H₂O(l) → 2NaAl(OH)₄(aq) + 3H₂(g)
This reaction must be handled with extreme caution due to the flammable nature of hydrogen gas.
4. Ester Hydrolysis
NaOH can also catalyze the hydrolysis of esters. This reaction breaks down esters into their constituent carboxylic acids and alcohols. This reaction is used in the production of various chemicals and in the analysis of esters.
Safety Precautions When Handling Sodium Hydroxide
Sodium hydroxide is corrosive and can cause severe burns to skin and eyes. Therefore, handling NaOH requires stringent safety measures:
-
Eye Protection: Always wear appropriate safety glasses or goggles when handling sodium hydroxide.
-
Protective Clothing: Wear gloves, lab coats, and other protective clothing to prevent skin contact.
-
Ventilation: Ensure adequate ventilation to minimize inhalation of dust or fumes.
-
First Aid: In case of skin or eye contact, immediately flush the affected area with copious amounts of water for at least 15 minutes and seek medical attention.
-
Storage: Store sodium hydroxide in airtight containers in a cool, dry place away from incompatible materials.
Improper handling can lead to severe injuries, emphasizing the need for caution and adherence to safety protocols.
Diverse Applications of Sodium Hydroxide
The strong base nature and other properties of sodium hydroxide make it invaluable across numerous industries:
1. Chemical Industry
-
Production of various chemicals: NaOH is a crucial reagent in the synthesis of numerous chemicals, including soaps, detergents, paper, textiles, and many others.
-
Neutralization reactions: It's extensively used to neutralize acidic waste streams and adjust pH levels in various chemical processes.
2. Food Industry
- Food processing: NaOH is used in food processing in controlled amounts for applications such as peeling fruits and vegetables, modifying food texture, and regulating pH. It's essential to note that proper regulatory compliance is required for any food application of sodium hydroxide.
3. Pulp and Paper Industry
- Pulp production: NaOH is a key component in the Kraft process, a widely used method for producing wood pulp for paper manufacturing. It helps in dissolving lignin, a complex polymer that binds wood fibers.
4. Metal Industries
-
Cleaning and degreasing metals: Sodium hydroxide solutions are effective in cleaning and degreasing metals before further processing.
-
Etching and surface treatment: It's also utilized in etching and surface treatment processes for metals.
5. Water Treatment
- pH control: NaOH is used to adjust the pH of water in water treatment plants, ensuring optimal conditions for various treatment processes.
6. Soap and Detergent Manufacturing
- Saponification: As discussed earlier, NaOH is a key ingredient in the saponification process for producing soaps.
7. Textile Industry
- Bleaching and dyeing: NaOH plays a role in bleaching and dyeing processes in textile manufacturing.
8. Oil and Gas Industry
- Drilling fluids: Sodium hydroxide is utilized in the preparation of drilling fluids used in oil and gas extraction.
Conclusion: The Importance of Sodium Hydroxide
Sodium hydroxide's status as a strong base is fundamental to its extensive applications across various sectors. Its remarkable properties – high solubility, reactivity, and complete dissociation – enable its use in numerous chemical reactions and industrial processes. However, its corrosive nature mandates strict adherence to safety protocols during handling and storage. Understanding the properties, reactions, and safety precautions associated with sodium hydroxide is essential for anyone working with this powerful chemical. Its versatility and significance in chemical and industrial processes underscore its critical role in modern society. Further research and development continue to expand the scope of its applications, making it an indispensable compound in chemical manufacturing and various other industries.
Latest Posts
Latest Posts
-
What Is The Lcm For 6 And 10
Apr 02, 2025
-
Which Organelles Supply Energy To The Cell
Apr 02, 2025
-
Why Is Density A Physical Property
Apr 02, 2025
-
Is Koh A Base Or Acid
Apr 02, 2025
-
Why Did Small States Object To The Virginia Plan
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Sodium Hydroxide Is A Strong Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
