Sigma And Pi Bonds In Co2
listenit
Mar 27, 2025 · 6 min read
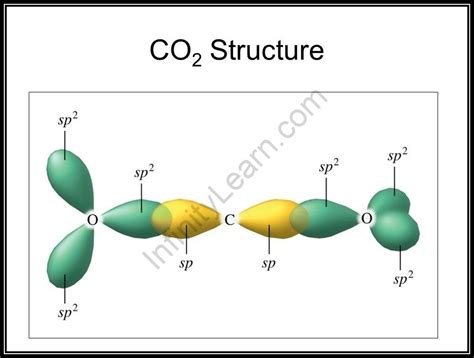
Table of Contents
Sigma and Pi Bonds in CO2: A Deep Dive into Molecular Structure and Properties
Carbon dioxide (CO2), a ubiquitous molecule in our atmosphere and a crucial component of the carbon cycle, presents a fascinating case study in chemical bonding. Understanding its structure, particularly the interplay between sigma (σ) and pi (π) bonds, is key to grasping its properties and behavior. This article will delve into the intricacies of CO2's bonding, exploring its implications for the molecule's linearity, reactivity, and overall significance.
Understanding Sigma and Pi Bonds
Before diving into the specifics of CO2, let's establish a clear understanding of sigma and pi bonds. These terms describe the types of covalent bonds formed between atoms by the overlapping of atomic orbitals.
Sigma (σ) Bonds: The Foundation of Covalent Bonding
A sigma bond is formed by the head-on overlap of atomic orbitals. This overlap is maximized along the internuclear axis, the imaginary line connecting the centers of the two bonded atoms. Sigma bonds are the strongest type of covalent bond and are fundamental to most molecular structures. They are characterized by free rotation around the bond axis. Simple single bonds are always sigma bonds.
Pi (π) Bonds: Adding Strength and Rigidity
Pi bonds are formed by the sideways overlap of atomic orbitals. Unlike sigma bonds, the electron density in a pi bond is concentrated above and below the internuclear axis. Pi bonds are generally weaker than sigma bonds. Crucially, pi bonds prevent free rotation around the bond axis, leading to greater rigidity in the molecule's structure. Double and triple bonds always include at least one pi bond in addition to a sigma bond.
The Molecular Structure of CO2: A Linear Arrangement
Carbon dioxide possesses a linear molecular geometry. This linearity is a direct consequence of the sigma and pi bonding within the molecule. Let's analyze the bonding scheme step-by-step:
Carbon's Hybridization: The Key to Understanding CO2's Geometry
Carbon, with its four valence electrons, undergoes sp hybridization in CO2. This hybridization involves the mixing of one 2s and two 2p orbitals to form two sp hybrid orbitals. These hybrid orbitals are oriented at 180 degrees to each other, laying the foundation for the linear arrangement of atoms. The remaining two 2p orbitals remain unhybridized and participate in pi bond formation.
Sigma Bond Formation in CO2
Each sp hybrid orbital on the carbon atom overlaps head-on with a 2p orbital from an oxygen atom, forming two strong sigma bonds. This accounts for two of the four bonds in the CO2 molecule.
Pi Bond Formation in CO2
The two unhybridized 2p orbitals on the carbon atom each overlap sideways with a 2p orbital from a respective oxygen atom. This results in the formation of two pi bonds. The presence of these pi bonds further strengthens the carbon-oxygen bonds and contributes to the molecule's overall stability.
Delving Deeper: Orbital Overlap and Electron Density
To fully appreciate the bonding in CO2, visualizing the orbital overlap and electron density is crucial.
Visualization of Sigma and Pi Overlap
Imagine the carbon atom at the center. The two sp hybrid orbitals extend outwards, each overlapping directly with a 2p orbital of an oxygen atom to form the sigma bonds. Concurrently, above and below the internuclear axis, the unhybridized 2p orbitals of carbon and oxygen overlap laterally, forming the two pi bonds. This results in a symmetrical distribution of electron density around the molecule.
Electron Density Distribution and Molecular Polarity
Despite the presence of polar carbon-oxygen bonds (oxygen being significantly more electronegative than carbon), the symmetrical arrangement of these bonds in the linear CO2 molecule results in a nonpolar molecule. The dipole moments of the individual C=O bonds cancel each other out, leading to a zero net dipole moment.
The Significance of Pi Bonds in CO2's Properties
The presence of pi bonds significantly influences several key properties of CO2:
1. Bond Strength and Stability:
The pi bonds contribute to the overall strength and stability of the carbon-oxygen double bonds. The combination of a sigma and a pi bond makes the C=O bond considerably stronger than a single C-O bond. This strength reflects in the molecule's high stability and relatively low reactivity under normal conditions.
2. Linear Geometry and Molecular Symmetry:
As previously discussed, the sp hybridization and the pi bond formation dictate the linear geometry of the molecule. This linearity contributes to the symmetry of the molecule and its nonpolar nature.
3. Reactivity and Chemical Behavior:
CO2's relative inertness under normal conditions stems partly from the stability imparted by the pi bonds. While not entirely unreactive, CO2 requires specific conditions or catalysts to participate in many chemical reactions. Its participation in photosynthesis, for example, requires complex enzymatic catalysis.
4. Infrared Spectroscopy and Vibrational Modes:
The presence of pi bonds, along with the overall molecular symmetry, influences the vibrational modes of CO2. This makes it readily identifiable using infrared spectroscopy, a technique that detects the absorption of infrared radiation by molecules due to specific vibrational modes.
CO2's Role in the Environment and Beyond
The properties of CO2, largely influenced by its bonding structure, are paramount to its role in the environment and various industrial applications:
Greenhouse Effect:
CO2's ability to absorb and re-emit infrared radiation makes it a potent greenhouse gas. This property, arising from its vibrational modes, contributes significantly to the Earth's climate. The linearity and symmetry of the molecule determine specific vibrational frequencies that resonate with infrared radiation.
Carbon Cycle:
CO2 plays a crucial role in the Earth’s carbon cycle. Through photosynthesis, plants convert CO2 into organic compounds, while respiration returns CO2 to the atmosphere. The stability and relatively unreactive nature of CO2 in its gaseous form allows it to persist in the atmosphere and participate in these vital processes.
Industrial Applications:
CO2 finds numerous applications in industry, including:
- Carbonated Beverages: Its solubility in water and its ability to form carbonic acid make it ideal for carbonating drinks.
- Fire Extinguishers: CO2's density and inert nature allow it to displace oxygen, suppressing combustion.
- Dry Ice: Solid CO2 (dry ice) is used as a refrigerant due to its ability to sublime (transition directly from solid to gas) at low temperatures.
- Chemical Feedstock: In certain industrial processes, CO2 serves as a reactant or a feedstock in the production of various chemicals.
Conclusion: A Powerful Molecule Defined by Its Bonds
The structure and properties of CO2 are deeply intertwined with its sigma and pi bonding. The sp hybridization of carbon, the formation of two sigma and two pi bonds, and the consequent linear geometry influence its stability, reactivity, and ultimately, its significant role in both natural and industrial processes. Understanding the intricacies of CO2's bonding scheme provides a critical foundation for comprehending its fundamental importance in chemistry, environmental science, and various industrial applications. Further research continues to uncover the multifaceted nature of this ubiquitous molecule and its interactions within complex systems.
Latest Posts
Latest Posts
-
1 Out Of 7 As A Percentage
Mar 30, 2025
-
What Element Has 4 Valence Electrons
Mar 30, 2025
-
The Theory Of Plate Tectonics States That
Mar 30, 2025
-
5 And 3 4 As A Decimal
Mar 30, 2025
-
How To Find The Equation Of Parallel Lines
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Sigma And Pi Bonds In Co2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
