Real Life Example Of Gay Lussac's Law
listenit
Mar 22, 2025 · 6 min read
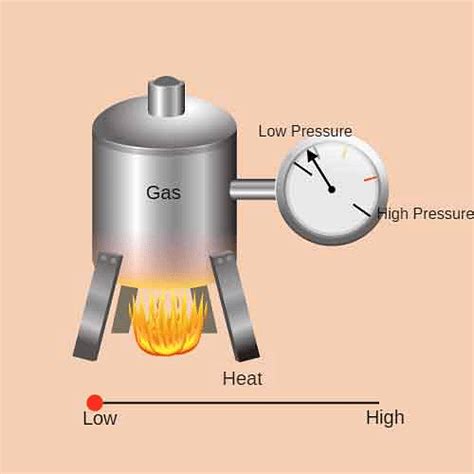
Table of Contents
Real-Life Examples of Gay-Lussac's Law: From Pressure Cookers to Balloons
Gay-Lussac's Law, also known as Amontons' Law, is a fundamental gas law that describes the relationship between the pressure and temperature of a gas when the volume is held constant. It states that the pressure of a gas is directly proportional to its absolute temperature, provided the volume remains constant. Mathematically, it's expressed as: P₁/T₁ = P₂/T₂, where P represents pressure and T represents absolute temperature (in Kelvin). While seemingly abstract, this law manifests itself in numerous everyday scenarios, impacting everything from cooking to weather patterns. Let's explore some compelling real-life examples:
Understanding the Fundamentals: Why Gay-Lussac's Law Matters
Before delving into specific examples, it's crucial to grasp the underlying principle. The law highlights the kinetic energy of gas molecules. As temperature increases, these molecules move faster, colliding more frequently and forcefully with the container walls. This increased collision rate translates directly to a higher pressure. Conversely, a decrease in temperature leads to slower-moving molecules, fewer collisions, and lower pressure. The constant volume ensures that the increased kinetic energy solely manifests as a pressure increase, rather than an expansion of the gas.
Real-World Applications: Illustrative Examples
Let's examine several practical scenarios where Gay-Lussac's Law plays a significant role:
1. Pressure Cookers: A Culinary Demonstration
Pressure cookers are a prime example of Gay-Lussac's Law in action. These ingenious kitchen appliances work by trapping steam within a sealed container. As the water boils, the temperature increases, leading to a rise in the pressure of the steam inside. This elevated pressure significantly increases the boiling point of water, allowing food to cook faster and more efficiently at higher temperatures. The pressure relief valve is crucial; it prevents the pressure from exceeding a safe level, preventing potential explosions. The relationship between the temperature of the steam and the internal pressure directly exemplifies Gay-Lussac's Law.
2. Aerosol Cans: Controlled Pressure Systems
Aerosol cans are another common application. These cans contain a propellant gas under pressure, usually a liquefied gas like butane or propane. When the valve is depressed, the gas expands rapidly, reducing its pressure and causing a cooling effect (demonstrating another gas law, Joule-Thomson effect). The initial pressure within the can is directly related to the temperature. On hot days, the pressure inside the can increases significantly, potentially leading to leaks or explosions if not designed to withstand the pressure fluctuations. Therefore, manufacturers carefully consider Gay-Lussac's Law during the design and safety testing of aerosol cans.
3. Hot Air Balloons: Buoyancy and Temperature
Hot air balloons provide a visually striking demonstration of Gay-Lussac's Law. The principle is simple: heating the air inside the balloon reduces its density, making it less dense than the surrounding cooler air. This density difference creates buoyancy, enabling the balloon to rise. The increase in temperature directly correlates to an increase in pressure inside the balloon (assuming the volume of the balloon remains relatively constant). The pilot controls altitude by adjusting the burner's intensity, thereby controlling the temperature and pressure within the balloon. A sudden, dramatic change in temperature, as might occur during a weather shift, will significantly impact the pressure inside the balloon, hence it’s crucial for pilot safety and effective operation.
4. Automobile Tires: Temperature and Pressure Fluctuations
Driving can heat up your car tires considerably. This temperature increase directly translates into an increase in tire pressure. Ignoring this phenomenon can lead to overinflation, resulting in uneven tire wear, reduced fuel efficiency, and potentially dangerous tire blowouts. Regular tire pressure checks are important to ensure safety and optimal performance. Checking the pressure when the tires are cold provides a more accurate reading before the effects of Gay-Lussac's Law come into play from driving.
5. Weather Balloons: Atmospheric Pressure Measurements
Weather balloons are used to gather atmospheric data at high altitudes. As the balloon ascends, the temperature of the surrounding air decreases. This temperature change affects the pressure inside the balloon. Scientists account for these pressure changes based on the altitude and predicted temperature profile of the atmosphere, enabling accurate measurements of atmospheric pressure at varying heights. The information gathered using these balloons is critical for weather forecasting. This process directly utilizes the principles of Gay-Lussac's Law.
6. Scuba Diving: Pressure Changes with Depth
Scuba diving presents another example. As divers descend, the pressure of the surrounding water increases significantly. The air within a diver's lungs also experiences this increased pressure; ignoring this would cause serious lung injuries. Divers must account for these pressure changes during their ascent and descent, equalizing pressure in their ears and lungs to maintain safety. The direct proportionality between pressure and temperature becomes relevant when considering the effects of breathing compressed air at various depths.
7. Industrial Processes: Maintaining Constant Pressure
Numerous industrial processes involve gases under controlled conditions. Maintaining constant pressure is often crucial in these processes. This is achieved by monitoring and adjusting temperature. Chemical reactions, manufacturing procedures, and power generation all frequently depend on accurately controlling gas pressure via temperature regulation, directly leveraging Gay-Lussac's Law.
Limitations and Considerations
While Gay-Lussac's Law provides a valuable framework, it's essential to acknowledge its limitations:
- Ideal Gas Assumption: The law is based on the ideal gas law, which assumes that gas molecules have negligible volume and do not interact with each other. Real gases deviate from this ideal behavior at high pressures and low temperatures.
- Constant Volume: The law strictly applies only when the volume of the gas remains constant. Any change in volume will invalidate the direct proportionality between pressure and temperature.
- Absolute Temperature: Temperature must always be expressed in Kelvin (K) for accurate calculations. Using Celsius or Fahrenheit will lead to incorrect results.
Conclusion: The Pervasiveness of Gay-Lussac's Law
Gay-Lussac's Law, despite its seemingly simple formulation, plays a crucial role in numerous aspects of our daily lives and various industrial applications. From the pressure cooker on your stove to the hot air balloon soaring through the sky, the relationship between pressure and temperature in a constant volume system is constantly at work, shaping our world in profound ways. Understanding this fundamental gas law offers valuable insights into how gases behave and provides a basis for understanding and controlling numerous real-world processes. By acknowledging both the applications and limitations of this law, we can appreciate its importance and apply its principles in a safe and effective manner.
Latest Posts
Latest Posts
-
45 Is What Percent Of 36
Mar 24, 2025
-
How Does A Igneous Rock Become A Sedimentary Rock
Mar 24, 2025
-
What Is 1 6 Of A Cup
Mar 24, 2025
-
Common Factors Of 16 And 18
Mar 24, 2025
-
What Volume Of Gas Is Evolved At Stp
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Real Life Example Of Gay Lussac's Law . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
