Protons Neutrons And Electrons Of Copper
listenit
Mar 26, 2025 · 6 min read
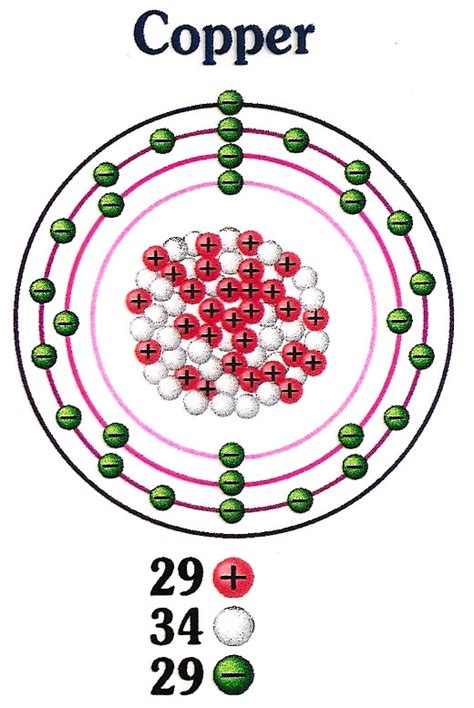
Table of Contents
Protons, Neutrons, and Electrons of Copper: A Deep Dive into Atomic Structure
Copper, a reddish-orange metal known for its excellent conductivity and malleability, plays a crucial role in our daily lives. From electrical wiring to plumbing to countless industrial applications, its properties are intrinsically linked to the fundamental building blocks of its atoms: protons, neutrons, and electrons. This article delves into the atomic structure of copper, exploring the characteristics and interactions of these subatomic particles and their impact on copper's unique properties.
Understanding the Atomic Structure of Copper
Copper (Cu), element number 29 on the periodic table, possesses a unique atomic structure that dictates its physical and chemical behavior. Each copper atom consists of a central nucleus, containing protons and neutrons, surrounded by a cloud of orbiting electrons. Let's examine each component individually:
Protons: The Defining Characteristic
The number of protons in an atom's nucleus determines its atomic number and defines the element. Copper's atomic number is 29, meaning every copper atom has 29 protons. These positively charged particles contribute significantly to the atom's overall mass and its positive charge. The proton's positive charge is crucial in balancing the negatively charged electrons, maintaining the atom's electrical neutrality in its ground state. The strong nuclear force binds protons and neutrons together within the nucleus, overcoming the electrostatic repulsion between the positively charged protons.
Neutrons: Stabilizing the Nucleus
Neutrons, as their name suggests, carry no electrical charge. They are found within the atomic nucleus alongside protons. The number of neutrons in a copper atom can vary, leading to different isotopes. The most common isotope of copper, ⁶³Cu, contains 34 neutrons (63 - 29 = 34), while another stable isotope, ⁶⁵Cu, has 36 neutrons. These neutrons play a crucial role in stabilizing the nucleus, preventing the electrostatic repulsion between protons from causing the nucleus to disintegrate. The presence of neutrons increases the strong nuclear force, which is a short-range attractive force that holds the nucleus together.
Electrons: The Conductors of Electricity
Electrons are negatively charged particles that orbit the nucleus in specific energy levels or shells. Copper has 29 electrons, mirroring its 29 protons to maintain electrical neutrality. These electrons are arranged in distinct electron shells, with the outermost shell, the valence shell, playing a crucial role in determining the element's chemical reactivity and electrical conductivity. Copper's electronic configuration is [Ar] 3d¹⁰ 4s¹. The single electron in the 4s subshell and the readily available 3d electrons are responsible for copper's excellent electrical conductivity. These electrons are relatively loosely bound to the atom and can move freely throughout the copper lattice, facilitating the flow of electric current. This electron mobility is a key characteristic that makes copper such a valuable material in electrical applications.
Isotopes of Copper: Variations in Neutron Count
As mentioned earlier, copper exists in two naturally occurring stable isotopes: ⁶³Cu and ⁶⁵Cu. These isotopes have the same number of protons (29) but differ in their neutron count. The abundance of ⁶³Cu is approximately 69%, while ⁶⁵Cu accounts for about 31% of naturally occurring copper.
Isotopic Abundance and Properties
The isotopic abundance of copper affects its average atomic mass, which is a weighted average of the masses of its isotopes. This average atomic mass is approximately 63.55 amu (atomic mass units). While the different isotopes have slightly varying masses, their chemical properties are essentially identical because the number of protons and electrons remains constant. The differences in neutron numbers primarily affect the physical properties like the nuclear stability and density, although these are subtle variations in the context of copper's overall properties.
Copper's Properties and Their Atomic Basis
Many of copper's remarkable properties are directly attributable to its unique atomic structure:
Electrical Conductivity: A Result of Mobile Electrons
The excellent electrical conductivity of copper is a direct consequence of its electronic configuration. The loosely bound electrons in the outermost shell are easily mobilized, allowing them to move freely under the influence of an electric field. This electron mobility is the foundation of copper's widespread use in electrical wiring and other conductive applications.
Thermal Conductivity: Related to Electron and Lattice Vibrations
Copper is also an excellent conductor of heat. This thermal conductivity is partly due to the mobility of its electrons, which can easily transfer kinetic energy. Lattice vibrations, the movement of copper atoms within the crystal lattice, also contribute to heat transfer. The energy from heat causes these atoms to vibrate more vigorously, transferring energy throughout the material.
Malleability and Ductility: The Role of Metallic Bonding
Copper's malleability (the ability to be shaped) and ductility (the ability to be drawn into wires) stem from the nature of metallic bonding. In a metallic bond, valence electrons are delocalized, meaning they are not associated with a particular atom but rather shared among all the atoms in the metallic lattice. This electron sea allows copper atoms to slide past one another without disrupting the overall structure, making it easily deformable.
Reddish-Orange Color: Interaction of Light and Electrons
The distinctive reddish-orange color of copper is a result of how its electrons interact with light. The electronic transitions within copper atoms selectively absorb certain wavelengths of light, while others are reflected. The reflected wavelengths constitute the color we perceive.
Applications of Copper: A Testament to its Properties
The unique combination of properties makes copper invaluable across a broad range of applications:
Electrical Wiring and Cables: Harnessing Conductivity
Copper's exceptional electrical conductivity makes it the material of choice for electrical wiring in buildings, vehicles, and countless electronic devices. Its ability to conduct electricity with minimal energy loss is essential for efficient power transmission.
Plumbing and Piping: Resistance to Corrosion
Copper's resistance to corrosion makes it suitable for plumbing systems, where it can withstand the effects of water and other chemicals. Copper pipes are durable and long-lasting, although issues relating to its susceptibility to certain chemicals are managed through appropriate engineering and maintenance practices.
Industrial Applications: A Versatile Metal
Copper's versatility extends to numerous industrial applications, including the manufacturing of alloys, heat exchangers, and various components in machinery. Its conductivity and other properties are essential in many industrial processes.
Coins and Medals: A Symbol of Value and Durability
Copper has a long history of use in coinage, valued for its durability and resistance to wear and tear. Its visual appeal and resistance to corrosion make it ideal for creating coins and medals.
Conclusion: The Significance of Subatomic Structure
The properties of copper, and indeed all materials, are fundamentally determined by the arrangement and behavior of their protons, neutrons, and electrons. Understanding the atomic structure of copper provides invaluable insights into its remarkable properties and its wide-ranging applications. The interplay between the positively charged nucleus and the negatively charged electrons defines copper's unique contributions to our technological world. From the efficient transmission of electricity to its durability in various applications, copper's success story is deeply rooted in the fundamental principles of atomic physics. Further research into the intricacies of copper's atomic structure may unlock even more possibilities for its use in future technologies. The ongoing exploration of materials science will undoubtedly continue to expand our understanding of copper and its potential.
Latest Posts
Latest Posts
-
Is Density A Physical Or Chemical Change
Mar 29, 2025
-
What Plane Divides The Body Into Anterior And Posterior Parts
Mar 29, 2025
-
How Many Electron Shells Does Carbon Have
Mar 29, 2025
-
Inverse Function Of X 3 X 2
Mar 29, 2025
-
Why Are Most Fossils Found In Sedimentary Rocks
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Protons Neutrons And Electrons Of Copper . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
