Protons Neutrons And Electrons In Copper
listenit
Mar 24, 2025 · 5 min read
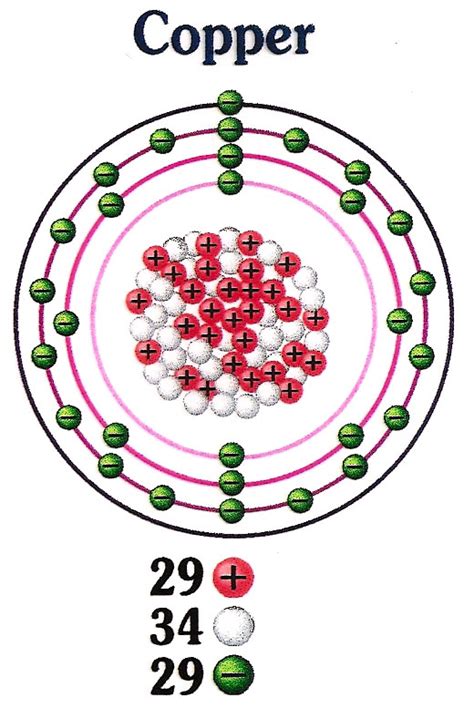
Table of Contents
Protons, Neutrons, and Electrons in Copper: A Deep Dive into Atomic Structure and Properties
Copper, a reddish-orange metal with exceptional electrical conductivity, plays a crucial role in numerous applications, from electrical wiring to plumbing. Understanding its properties necessitates delving into the fundamental building blocks of its atomic structure: protons, neutrons, and electrons. This comprehensive article explores the roles of these subatomic particles in defining copper's unique characteristics, from its electrical conductivity to its malleability.
Understanding the Atomic Structure of Copper
Copper's atomic number is 29, which signifies the number of protons residing in its nucleus. The nucleus also contains neutrons, whose number can vary, leading to different isotopes of copper. Surrounding the nucleus is a cloud of electrons, occupying specific energy levels or shells. These electrons are the key players in determining copper's chemical and physical behavior.
The Role of Protons
The protons, each carrying a single positive charge, determine the element's identity. In copper, the 29 protons define it as copper and differentiate it from other elements on the periodic table. The positive charge of the protons is crucial for maintaining the overall electrical neutrality of the atom, balanced by the negative charge of the electrons. The number of protons in the nucleus directly influences copper's interactions with other atoms and its place within the periodic table's trends. The strong nuclear force binds the protons together within the nucleus, despite their mutual electrostatic repulsion.
The Significance of Neutrons
Copper's nucleus also houses neutrons, particles with no net electrical charge. While protons determine the element's identity, neutrons contribute significantly to its stability and mass. The most common isotopes of copper are <sup>63</sup>Cu and <sup>65</sup>Cu. These isotopes differ in their neutron count; <sup>63</sup>Cu has 34 neutrons, and <sup>65</sup>Cu has 36 neutrons. The differing neutron numbers contribute to slight variations in the isotopes' atomic mass and, to a lesser extent, their properties. The strong nuclear force, alongside the electromagnetic force, is responsible for holding protons and neutrons together within the incredibly dense nucleus.
The Behavior of Electrons and Their Influence on Copper's Properties
Electrons, each possessing a single negative charge, occupy specific energy levels or shells surrounding the copper nucleus. These energy levels are quantized, meaning electrons can only exist at certain discrete energy states. Copper's electron configuration is [Ar] 3d<sup>10</sup> 4s<sup>1</sup>. This configuration explains many of its physical and chemical properties.
-
Electrical Conductivity: The outermost electron (in the 4s orbital) is relatively loosely bound to the atom. This loosely bound electron can easily move from one atom to another, facilitating the flow of electrical current. This high mobility of the electron is responsible for copper's excellent electrical conductivity—a property extensively utilized in electrical wiring and various electronic components.
-
Thermal Conductivity: Similar to its electrical conductivity, copper's high thermal conductivity stems from the mobility of its electrons. The electrons readily transfer kinetic energy, effectively conducting heat throughout the material. This property finds applications in heat exchangers and other thermal management systems.
-
Malleability and Ductility: The metallic bonding in copper, arising from the delocalized electrons, allows copper atoms to slide past one another without significantly disrupting the metallic structure. This makes copper highly malleable (easily shaped) and ductile (easily drawn into wires). This property is crucial for its use in various metalworking applications.
-
Chemical Reactivity: While relatively unreactive compared to some other metals, copper can participate in chemical reactions, forming various compounds. The single valence electron in the 4s orbital is involved in these reactions, often leading to the formation of copper(I) and copper(II) ions.
Isotopes of Copper: A Closer Look
As mentioned previously, copper has two naturally occurring isotopes: <sup>63</sup>Cu and <sup>65</sup>Cu. These isotopes differ in their neutron count but share the same number of protons (29). Their abundance in nature is approximately 69.17% for <sup>63</sup>Cu and 30.83% for <sup>65</sup>Cu. This isotopic variation minimally affects the overall properties of copper but is important to consider in certain applications, like nuclear physics and isotopic tracing.
Copper's Role in Various Applications
Copper's unique combination of properties makes it a versatile material utilized across numerous applications. Its high electrical conductivity is pivotal in:
-
Electrical Wiring: Copper wires are ubiquitous in electrical systems worldwide, facilitating the efficient transmission of electricity.
-
Electronic Components: Copper is employed in various electronic components, including printed circuit boards and integrated circuits, due to its excellent conductivity and ability to be easily formed into intricate shapes.
-
Transformers and Motors: The high conductivity and low resistance of copper are essential in the construction of efficient transformers and electric motors.
Beyond its electrical applications, copper's other properties find use in:
-
Plumbing: Copper pipes are widely used in plumbing systems due to their corrosion resistance and durability.
-
Coins and Medals: Copper's inherent aesthetic appeal and resistance to corrosion make it a suitable material for minting coins and producing medals.
-
Heat Exchangers: Copper's exceptional thermal conductivity finds application in heat exchangers, enabling efficient heat transfer in various industrial processes.
-
Alloying: Copper is used as a base metal in many alloys, such as brass (copper and zinc) and bronze (copper and tin), which enhance the material's properties for specialized applications.
Conclusion: The Interplay of Subatomic Particles in Shaping Copper's Properties
The interplay of protons, neutrons, and electrons within the copper atom is fundamental to understanding its remarkable properties. The 29 protons establish its identity as copper, while the varying neutron counts give rise to its isotopes. The loosely bound outer electrons are responsible for copper's exceptional electrical and thermal conductivity, as well as its malleability and ductility. These properties make copper an indispensable material in countless applications, shaping our modern world in significant ways. Further research into the detailed behavior of electrons in copper and its interaction with other elements will continue to unlock new and exciting applications for this important metal. Understanding the fundamental atomic structure is critical for further innovations and advancements in materials science and engineering. The ongoing exploration of copper's properties and its potential for new technological applications underlines the enduring importance of understanding the basics of atomic structure.
Latest Posts
Latest Posts
-
What Is The Lcm Of 14 And 21
Mar 29, 2025
-
Is Electron Affinity The Same As Electronegativity
Mar 29, 2025
-
What Is The Expected Response To The Triceps Jerk Reflex
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Protons Neutrons And Electrons In Copper . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
