Is Electron Affinity The Same As Electronegativity
listenit
Mar 29, 2025 · 5 min read
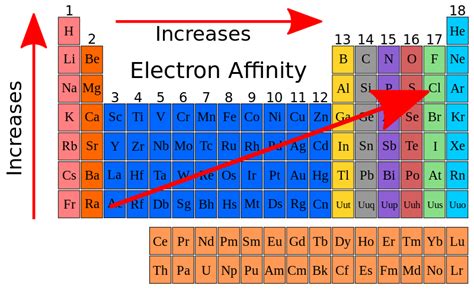
Table of Contents
Is Electron Affinity the Same as Electronegativity?
While both electron affinity and electronegativity relate to an atom's attraction for electrons, they are distinct concepts with crucial differences. Understanding these differences is vital for comprehending chemical bonding and reactivity. This article delves deep into the definitions, measurements, trends, and applications of both electron affinity and electronegativity, highlighting their similarities and, more importantly, their key distinctions.
Defining Electron Affinity
Electron affinity (EA) is the energy change that occurs when an atom in the gaseous phase gains an electron. This process can either release energy (exothermic, negative EA value) or require energy (endothermic, positive EA value). A high electron affinity indicates that the atom readily accepts an electron, releasing a significant amount of energy in the process. Conversely, a low or positive electron affinity signifies that the atom is less inclined to accept an electron and may even require energy input for electron acceptance.
Factors Affecting Electron Affinity:
Several factors influence an atom's electron affinity:
-
Effective Nuclear Charge: A higher effective nuclear charge (the net positive charge experienced by the outermost electrons) leads to a stronger attraction for incoming electrons, resulting in a higher electron affinity.
-
Atomic Size: Smaller atoms generally have higher electron affinities because the incoming electron is closer to the nucleus and experiences a stronger attractive force.
-
Electron Shell Configuration: Half-filled and completely filled electron subshells are particularly stable. Atoms with these configurations exhibit lower electron affinities than those with nearly filled subshells. Adding an electron disrupts this stability.
-
Electron-Electron Repulsion: Adding an electron to an already negatively charged atom or ion increases electron-electron repulsion, reducing the electron affinity. This effect is more pronounced in larger atoms where electrons are further apart.
Defining Electronegativity
Electronegativity (χ) is a measure of an atom's ability to attract shared electrons in a chemical bond. Unlike electron affinity, which focuses on an isolated atom gaining an electron, electronegativity describes the behavior of an atom within a bond. It's a relative property, meaning it's compared to other atoms. The higher the electronegativity value, the stronger an atom attracts bonding electrons towards itself.
Factors Affecting Electronegativity:
Similar to electron affinity, electronegativity is influenced by:
-
Effective Nuclear Charge: A greater effective nuclear charge results in a higher electronegativity, as the nucleus more strongly attracts bonding electrons.
-
Atomic Size: Smaller atoms generally exhibit higher electronegativity due to the closer proximity of the bonding electrons to the nucleus.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. A greater shielding effect reduces electronegativity.
Key Differences Between Electron Affinity and Electronegativity
While both properties relate to an atom's attraction for electrons, several key distinctions set them apart:
| Feature | Electron Affinity | Electronegativity |
|---|---|---|
| Definition | Energy change upon gaining an electron (gas phase) | Ability to attract shared electrons in a chemical bond |
| Nature | Absolute property (energy value) | Relative property (compared to other atoms) |
| Process | Single electron gain by an isolated atom | Attraction of shared electrons in a bond |
| Measurement | Measured experimentally (e.g., photoelectron spectroscopy) | Calculated using various scales (e.g., Pauling scale, Mulliken scale) |
| Units | kJ/mol or eV | Dimensionless (scale-dependent) |
| Focus | Isolated atom | Atom within a chemical bond |
Trends in Electron Affinity and Electronegativity Across the Periodic Table
Both electron affinity and electronegativity exhibit predictable trends across the periodic table:
Electron Affinity:
-
Increases across a period (left to right): Generally, electron affinity increases across a period due to the increasing effective nuclear charge. However, exceptions exist due to electronic configurations (e.g., group 15 elements).
-
Generally decreases down a group (top to bottom): Decreases as atomic size increases, resulting in weaker attraction to the incoming electron.
Electronegativity:
-
Increases across a period (left to right): Higher effective nuclear charge leads to stronger attraction of shared electrons. Fluorine is the most electronegative element.
-
Decreases down a group (top to bottom): Increasing atomic size reduces the attraction for shared electrons.
Applications of Electron Affinity and Electronegativity
Both electron affinity and electronegativity are crucial for understanding various chemical phenomena:
Electron Affinity:
-
Predicting the formation of anions: High electron affinity suggests a greater likelihood of an atom forming a stable anion.
-
Understanding redox reactions: Electron affinity plays a role in determining the ease of reduction (gaining electrons) in redox processes.
-
Materials science: Understanding electron affinity helps in designing materials with specific electronic properties.
Electronegativity:
-
Predicting bond polarity: The difference in electronegativity between atoms in a bond determines the bond's polarity (ionic or covalent). A large difference suggests an ionic bond, while a small difference suggests a covalent bond.
-
Determining molecular polarity: Molecular polarity depends on the individual bond polarities and the molecule's geometry.
-
Understanding chemical reactivity: Electronegativity helps predict the reactivity of molecules and their participation in various chemical reactions.
-
Organic chemistry: Electronegativity is vital in predicting the reactivity of functional groups and the stability of reaction intermediates.
Misconceptions and Clarifications
A common misconception is that electron affinity and electronegativity are directly proportional. While both are related to the attraction of electrons, the nature of this attraction differs significantly. Electron affinity refers to the energy change when a single electron is added to an isolated gaseous atom, whereas electronegativity describes the ability of an atom within a bond to attract shared electrons. They are not directly interchangeable.
Conclusion
While both electron affinity and electronegativity reflect an atom's tendency to attract electrons, their definitions, measurements, and applications are distinct. Electron affinity describes the energy change associated with gaining a single electron by an isolated gaseous atom, while electronegativity focuses on the atom's ability to attract shared electrons within a chemical bond. Both are powerful tools for predicting and understanding chemical behavior and are essential concepts in chemistry and related fields. Understanding their differences is crucial for accurate prediction of chemical properties and reactivity. The trends across the periodic table provide a valuable framework for predicting these properties, even though exceptions may exist due to complexities in electron configurations and shielding effects. The applications highlighted emphasize their importance across various branches of chemistry and materials science.
Latest Posts
Latest Posts
-
How Much Is Half A Gallon In Cups
Mar 31, 2025
-
What Is 5 5 As A Decimal
Mar 31, 2025
-
Is Gasoline Evaporating A Chemical Change
Mar 31, 2025
-
What Is Half Of A Mile In Feet
Mar 31, 2025
-
Common Factors Of 20 And 36
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Electron Affinity The Same As Electronegativity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
