Oxidation State Of O In H2o
listenit
Mar 29, 2025 · 6 min read
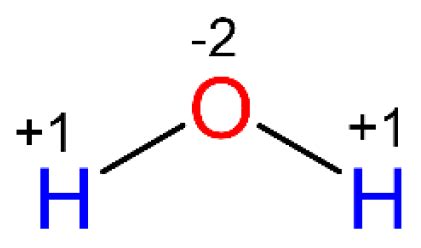
Table of Contents
The Oxidation State of Oxygen in Water (H₂O): A Deep Dive
The seemingly simple water molecule, H₂O, offers a fascinating study in chemical bonding and oxidation states. While the concept of oxidation state might seem straightforward, a deeper examination reveals nuances and subtleties that are crucial for understanding various chemical processes. This article provides a comprehensive exploration of the oxidation state of oxygen in water, delving into its determination, significance, and implications in diverse chemical contexts.
Understanding Oxidation States
Before diving into the specifics of oxygen in water, let's establish a foundational understanding of oxidation states. Oxidation state, also known as oxidation number, is a number assigned to an atom in a molecule or ion that represents the hypothetical charge that atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial concept in redox chemistry (reduction-oxidation reactions), enabling us to track electron transfer during chemical reactions.
While it's a hypothetical charge, the oxidation state provides a valuable tool for:
- Balancing redox reactions: Determining the change in oxidation states helps in balancing complex redox equations.
- Predicting reaction tendencies: Oxidation states can help predict the likelihood of a substance acting as an oxidizing or reducing agent.
- Understanding chemical bonding: The oxidation state offers insights into the nature of chemical bonds within a molecule.
Several rules govern the assignment of oxidation states, which are applied systematically:
- Free elements: Atoms in their elemental form have an oxidation state of 0.
- Monatomic ions: The oxidation state of a monatomic ion is equal to its charge.
- Fluorine: Fluorine, the most electronegative element, always has an oxidation state of -1 in its compounds.
- Oxygen: Oxygen usually has an oxidation state of -2 in its compounds, except in peroxides (e.g., H₂O₂) where it's -1, and in compounds with fluorine (e.g., OF₂) where it's +2.
- Hydrogen: Hydrogen usually has an oxidation state of +1 in its compounds, except in metal hydrides (e.g., NaH) where it's -1.
- Sum of oxidation states: The sum of oxidation states of all atoms in a neutral molecule is zero, while in a polyatomic ion, it equals the charge of the ion.
Determining the Oxidation State of Oxygen in H₂O
Applying these rules to water (H₂O) is relatively straightforward. We know that:
- Hydrogen (H): Typically has an oxidation state of +1.
- Water (H₂O): Is a neutral molecule, meaning the sum of oxidation states must be 0.
Let's represent the oxidation state of oxygen as 'x'. Using the sum of oxidation states rule:
2(+1) + x = 0
Solving for x:
x = -2
Therefore, the oxidation state of oxygen in water is -2. This aligns with the general rule for oxygen's oxidation state in most of its compounds.
Significance of Oxygen's Oxidation State in Water
The -2 oxidation state of oxygen in water is highly significant for several reasons:
-
Water's stability: The stable nature of water is partly attributed to the strong electronegativity of oxygen and the resulting strong O-H bonds. The -2 oxidation state reflects this electronegativity and the tendency of oxygen to attract electrons towards itself.
-
Water's role in redox reactions: Water can act as both an oxidizing and reducing agent, depending on the reaction conditions and the other reactants involved. Understanding the oxidation state of oxygen helps in predicting its role in these reactions. For instance, in the reaction of water with a strong reducing agent like an alkali metal, water can act as an oxidizing agent, itself getting reduced to hydrogen gas. Conversely, in the presence of a strong oxidizing agent like fluorine, it can act as a reducing agent.
-
Environmental chemistry: The oxidation state of oxygen plays a crucial role in various environmental processes, including:
- Aerobic respiration: Oxygen's high electronegativity and tendency to accept electrons make it a vital electron acceptor in aerobic respiration, a process that underpins most life on Earth.
- Water purification: Oxidation processes, which often involve oxygen, are vital for purifying water from various pollutants.
-
Industrial processes: Many industrial processes rely on the redox properties of water and its components, where the understanding of oxygen's oxidation state is essential. For instance, the electrolysis of water utilizes the oxidation and reduction of water to produce hydrogen and oxygen gas.
Exceptions and Special Cases
While -2 is the most common oxidation state for oxygen, exceptions exist, particularly in peroxides and compounds with more electronegative elements like fluorine.
Peroxides (e.g., H₂O₂)
In peroxides, oxygen atoms form a single bond with each other (O-O). Due to the similar electronegativity of the two oxygen atoms, the electron pair in the O-O bond is shared equally, leading to an oxidation state of -1 for each oxygen atom.
Oxygen Fluorides (e.g., OF₂)
Fluorine, being the most electronegative element, pulls electrons away from oxygen. In oxygen difluoride (OF₂), oxygen exhibits an unusual +2 oxidation state, demonstrating the exception to the rule that oxygen is almost always negative.
Advanced Concepts and Applications
The seemingly simple oxidation state of oxygen in water opens doors to more complex concepts:
-
Formal charge vs. oxidation state: While related, formal charge and oxidation state differ. Formal charge considers the electron distribution in a molecule based on assigning electrons equally between bonded atoms, irrespective of electronegativity differences. Oxidation state, on the other hand, accounts for electronegativity differences and assigns electrons to the more electronegative atom.
-
Bond polarity and electronegativity: The electronegativity difference between oxygen and hydrogen in water results in polar O-H bonds, where oxygen carries a partial negative charge and hydrogen carries a partial positive charge. This polarity is crucial for water's unique properties, such as its high surface tension and ability to act as a solvent for many ionic compounds.
-
Redox potential: Redox potential, a measure of a substance's ability to gain or lose electrons, is linked to oxidation states. Water's redox potential is influenced by the oxidation state of oxygen and the presence of other species in solution.
Conclusion
The oxidation state of oxygen in water, seemingly a simple -2, underpins a wealth of chemical phenomena. Understanding this oxidation state is fundamental to comprehending the nature of water's bonding, its role in redox reactions, and its significance in various chemical and environmental processes. From the stability of water to its role in industrial applications and environmental chemistry, the -2 oxidation state of oxygen is a cornerstone of chemical understanding, demonstrating the power of seemingly simple concepts in explaining complex natural occurrences. Exploring this seemingly basic concept opens a door to advanced chemical concepts, emphasizing the interconnectivity and complexity within even the most seemingly simple chemical structures. This deeper understanding highlights the importance of rigorous chemical analysis and its importance in different areas of study, emphasizing the importance of fundamental principles in expanding knowledge in the scientific world.
Latest Posts
Latest Posts
-
The Lock And Key Mechanism Refers To
Mar 31, 2025
-
What Percent Of 25 Is 4
Mar 31, 2025
-
Number Of Protons Neutrons And Electrons In Carbon
Mar 31, 2025
-
What Percentage Is 4 Out Of 20
Mar 31, 2025
-
The Mercalli Scale Is A Scale From
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Oxidation State Of O In H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
