Of The Following Elements Which Has The Highest Electronegativity
listenit
Mar 28, 2025 · 5 min read
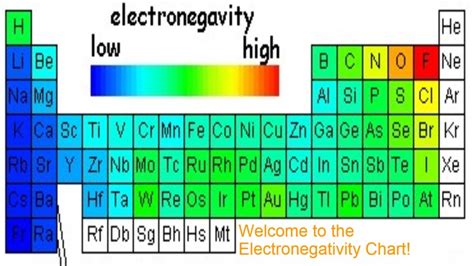
Table of Contents
Of the Following Elements, Which Has the Highest Electronegativity? A Deep Dive into Electronegativity Trends
Electronegativity, a fundamental concept in chemistry, quantifies an atom's ability to attract electrons within a chemical bond. Understanding electronegativity is crucial for predicting the nature of chemical bonds (ionic, covalent, polar covalent), molecular polarity, and reactivity. This article will explore electronegativity, its trends across the periodic table, and definitively answer the question: which element boasts the highest electronegativity? We will also delve into the factors that influence electronegativity and its practical applications.
Understanding Electronegativity
Electronegativity isn't a directly measurable property like mass or charge. Instead, it's a relative value, typically expressed on the Pauling scale, with values ranging from 0.7 (for cesium) to 4.0 (for fluorine). The higher the electronegativity value, the stronger an atom's pull on shared electrons in a bond.
Key Factors Influencing Electronegativity:
-
Nuclear Charge: A greater number of protons in the nucleus leads to a stronger positive charge, attracting electrons more effectively. This is why electronegativity generally increases across a period (left to right) in the periodic table.
-
Atomic Radius: Smaller atoms have electrons closer to the nucleus, experiencing a stronger attractive force. Therefore, electronegativity generally decreases down a group (top to bottom) in the periodic table.
-
Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by valence electrons, thus decreasing electronegativity.
Trends in Electronegativity Across the Periodic Table
Understanding the periodic trends is essential for predicting relative electronegativities.
Across a Period (Left to Right):
Electronegativity generally increases from left to right across a period. This is primarily because the nuclear charge increases while the atomic radius remains relatively constant. Electrons are added to the same principal energy level, experiencing minimal shielding from inner electrons. The increasing positive charge pulls the valence electrons more strongly.
Down a Group (Top to Bottom):
Electronegativity generally decreases from top to bottom within a group. As you move down a group, the atomic radius increases significantly. The increased distance between the nucleus and the valence electrons weakens the attractive force, leading to lower electronegativity. Furthermore, the increased number of inner electrons enhances the shielding effect, further reducing the effective nuclear charge.
The Element with the Highest Electronegativity: Fluorine
After examining the trends, it becomes clear: fluorine (F) possesses the highest electronegativity, with a value of approximately 4.0 on the Pauling scale.
This exceptional electronegativity stems from a combination of factors:
-
High Nuclear Charge: Fluorine has a relatively high nuclear charge for its size.
-
Small Atomic Radius: Fluorine is a very small atom, placing its valence electrons exceptionally close to the nucleus.
-
Minimal Shielding: Fluorine has only two inner electrons, providing minimal shielding to its valence electrons.
These factors combine to create an incredibly strong attractive force on electrons involved in chemical bonds, making fluorine the most electronegative element.
Comparing Electronegativities: A Case Study
Let's compare the electronegativities of several elements to illustrate the trends and highlight fluorine's dominance.
| Element | Electronegativity (Pauling Scale) | Period | Group |
|---|---|---|---|
| Fluorine (F) | 4.0 | 2 | 17 |
| Oxygen (O) | 3.5 | 2 | 16 |
| Chlorine (Cl) | 3.0 | 3 | 17 |
| Nitrogen (N) | 3.0 | 2 | 15 |
| Bromine (Br) | 2.8 | 4 | 17 |
| Carbon (C) | 2.5 | 2 | 14 |
| Hydrogen (H) | 2.1 | 1 | 1 |
| Sodium (Na) | 0.9 | 3 | 1 |
| Cesium (Cs) | 0.7 | 6 | 1 |
This table demonstrates the clear trend: electronegativity increases across a period (e.g., from Na to F) and decreases down a group (e.g., from F to Cl to Br). Fluorine consistently stands out as the most electronegative element.
Applications of Electronegativity
Understanding electronegativity is vital in several areas of chemistry:
Predicting Bond Type:
-
Ionic Bonds: When the difference in electronegativity between two atoms is large (typically greater than 1.7), electrons are transferred from the less electronegative atom to the more electronegative atom, forming ions and an ionic bond. For example, the bond between sodium (Na) and chlorine (Cl) in NaCl is ionic.
-
Covalent Bonds: When the electronegativity difference is small (typically less than 0.5), electrons are shared relatively equally between atoms, resulting in a nonpolar covalent bond. For instance, the bond between two hydrogen atoms in H₂ is nonpolar covalent.
-
Polar Covalent Bonds: When the electronegativity difference is intermediate (between 0.5 and 1.7), electrons are shared unequally, creating a polar covalent bond with a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. Water (H₂O) is a classic example of a molecule with polar covalent bonds.
Predicting Molecular Polarity:
Molecular polarity is determined by both the polarity of individual bonds and the molecule's geometry. Electronegativity helps predict bond polarity, a crucial factor in determining overall molecular polarity, which affects properties like solubility and boiling point.
Understanding Reactivity:
Electronegativity influences the reactivity of elements. Highly electronegative atoms tend to readily accept electrons, while atoms with low electronegativity readily donate electrons.
Beyond the Pauling Scale: Other Electronegativity Scales
While the Pauling scale is the most widely used, other scales exist, including the Mulliken scale and the Allred-Rochow scale. These scales utilize different approaches but aim to quantify the same fundamental property: an atom's tendency to attract electrons in a chemical bond. While the exact numerical values differ between scales, the overall trends and relative electronegativities remain consistent.
Conclusion
In conclusion, fluorine (F) holds the title of the element with the highest electronegativity. This exceptional property arises from a combination of a high nuclear charge, small atomic radius, and minimal shielding. Understanding electronegativity trends and their impact on bond type, molecular polarity, and reactivity is fundamental to a comprehensive understanding of chemical bonding and reactivity. The concept remains crucial in various chemical applications and serves as a cornerstone of chemical prediction and analysis. Further exploration into the nuances of electronegativity and its applications will continue to deepen our understanding of the fundamental forces governing the behavior of matter.
Latest Posts
Latest Posts
-
80 Is What Percent Of 40
Mar 31, 2025
-
Is The Square Root Of 5 A Rational Number
Mar 31, 2025
-
Graph 4x 7 Greater Than X 13
Mar 31, 2025
-
8 Quarts Equals How Many Pints
Mar 31, 2025
-
How To Find Width Of Rectangular Prism
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Of The Following Elements Which Has The Highest Electronegativity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
