Number Of Valence Electrons In Phosphorus
listenit
Mar 16, 2025 · 6 min read
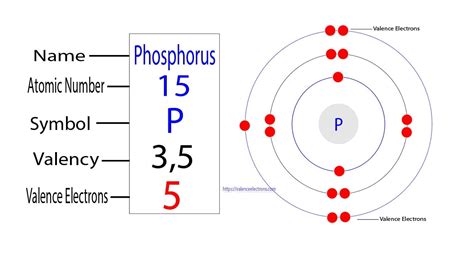
Table of Contents
The Enigmatic Valence Electrons of Phosphorus: A Deep Dive
Phosphorus, a nonmetal element crucial to life as we know it, holds a fascinating position in the periodic table. Its chemical behavior, particularly its bonding capabilities, is directly tied to its number of valence electrons. Understanding this fundamental aspect is key to unlocking its diverse roles in everything from DNA to fertilizers. This article will delve into the intricacies of phosphorus's valence electrons, exploring its electronic configuration, bonding characteristics, and the implications for its chemical reactivity.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we focus specifically on phosphorus, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the primary players in chemical bonding, determining how an atom interacts with other atoms to form molecules and compounds. The number of valence electrons directly influences an element's reactivity, oxidation state, and the types of bonds it can form (ionic, covalent, or metallic). Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gases with their filled valence shells (octet rule, although there are exceptions).
Determining the Number of Valence Electrons in Phosphorus
Phosphorus (P), with an atomic number of 15, possesses 15 electrons in total. To determine its valence electrons, we need to examine its electronic configuration. Using the Aufbau principle and Hund's rule, we can distribute these electrons across the various energy levels and orbitals:
1s² 2s² 2p⁶ 3s² 3p³
The electronic configuration shows that the first two energy levels (n=1 and n=2) are completely filled. However, the third energy level (n=3) is only partially filled. The valence electrons are those in the outermost, highest energy level. In this case, it's the third energy level, containing 5 electrons (2 from the 3s orbital and 3 from the 3p orbitals).
Therefore, phosphorus has 5 valence electrons.
Visualizing Phosphorus's Electron Configuration
Imagine the phosphorus atom like a layered onion. The innermost layer (n=1) has 2 electrons tightly bound to the nucleus. The next layer (n=2) holds 8 electrons, while the outermost layer (n=3), the valence shell, contains 5 electrons. These 5 valence electrons are the ones most readily involved in chemical interactions. This outer layer's incompleteness drives phosphorus's reactivity, leading it to seek ways to complete this outer shell.
The Chemical Implications of Phosphorus's 5 Valence Electrons
The presence of 5 valence electrons dictates phosphorus's diverse chemical behavior and its ability to form various compounds. Let's explore some key implications:
1. Covalent Bonding Prowess:
With 5 valence electrons, phosphorus readily forms covalent bonds. It can share electrons with other atoms to achieve a stable octet configuration. This results in the formation of various phosphorus-containing molecules, such as:
- Phosphine (PH₃): Phosphorus shares its three unpaired 3p electrons with three hydrogen atoms, forming three covalent bonds.
- Phosphorous trichloride (PCl₃): Here, phosphorus shares its three unpaired electrons with three chlorine atoms.
- Phosphorous pentachloride (PCl₅): This molecule is a fascinating exception to the octet rule. Phosphorus expands its valence shell to accommodate 10 electrons, forming five covalent bonds with five chlorine atoms. This demonstrates the ability of phosphorus, and other elements in the third row and beyond, to exceed the octet rule due to the availability of empty d-orbitals.
2. Oxidation States and Redox Reactions:
The five valence electrons allow phosphorus to exhibit a range of oxidation states, from -3 (e.g., in phosphides) to +5 (e.g., in phosphate). This versatility enables phosphorus to participate in numerous redox (reduction-oxidation) reactions, where electrons are transferred between atoms. These reactions are fundamental to many biological and industrial processes.
3. Formation of Polyatomic Ions:
Phosphorus's ability to form covalent bonds extends to the formation of polyatomic ions – charged molecules containing multiple atoms. Perhaps the most important example is the phosphate ion (PO₄³⁻). In this ion, phosphorus is covalently bonded to four oxygen atoms, carrying a 3- charge. Phosphate ions are essential components of DNA, RNA, and ATP (adenosine triphosphate), the energy currency of cells.
4. Allotropes of Phosphorus:
The different ways phosphorus atoms can bond to each other lead to the existence of several allotropes – different structural forms of the same element. White phosphorus, red phosphorus, and black phosphorus are three well-known allotropes, each with distinct physical and chemical properties due to variations in their bonding arrangements. These variations are a direct consequence of the electron configuration and the versatile bonding capabilities afforded by the 5 valence electrons.
Phosphorus's Role in Biological Systems: A Valence Electron Perspective
Phosphorus's unique bonding properties, stemming directly from its 5 valence electrons, are paramount to its crucial role in various biological systems.
1. Energy Transfer and Storage:
ATP, the primary energy-carrying molecule in living organisms, incorporates phosphate groups linked through high-energy phosphoanhydride bonds. The breaking of these bonds releases energy that drives numerous cellular processes. This critical function relies directly on the bonding characteristics made possible by phosphorus's valence electrons.
2. Nucleic Acid Structure:
The backbone of DNA and RNA, the carriers of genetic information, is built upon a sugar-phosphate backbone. The phosphate groups link the sugar molecules, forming a continuous chain. Without phosphorus's capacity to form these stable bonds, the intricate structure of our genetic material would be impossible.
3. Phospholipids and Cell Membranes:
Phospholipids, major components of cell membranes, possess a phosphate group in their head. This hydrophilic (water-loving) head interacts with the aqueous environment, while the hydrophobic (water-fearing) tails form the lipid bilayer structure of the membrane. The properties of the phosphate group, directly linked to its bonding capacity arising from its 5 valence electrons, are crucial for maintaining cell membrane integrity.
Beyond Biology: Industrial Applications of Phosphorus
The versatility of phosphorus's bonding, a consequence of its 5 valence electrons, extends far beyond biological systems. It plays a vital role in various industrial applications:
- Fertilizers: Phosphorus is a critical component of fertilizers, providing a vital nutrient for plant growth. Phosphate-containing compounds are essential for plant development, contributing to increased crop yields.
- Detergents: Phosphate compounds were once widely used in detergents but have been reduced due to environmental concerns. They are effective water softeners and help remove dirt and grime.
- Flame Retardants: Certain phosphorus compounds are utilized as flame retardants, inhibiting the spread of fires.
- Metallurgy: Phosphorus is added to some alloys to enhance their properties.
Conclusion: The Significance of Phosphorus's Valence Electrons
The number of valence electrons an atom possesses is fundamental to understanding its chemical behavior. For phosphorus, with its 5 valence electrons, this characteristic governs its ability to form diverse covalent bonds, exhibit a wide range of oxidation states, create essential biological molecules like ATP and nucleic acids, and play a crucial role in various industrial applications. Understanding this core principle unveils the key to phosphorus's unique and vital contribution to the world around us, from the intricate workings of life itself to the materials that shape our modern world. Further exploration of its chemistry continues to reveal new insights into this essential element, highlighting the profound impact of its electron configuration on its multifaceted roles.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 15 And 9
Mar 16, 2025
-
What Is The Electron Configuration For N
Mar 16, 2025
-
How Much 67 Kg In Pounds
Mar 16, 2025
-
How To Solve X 3 X 2 X
Mar 16, 2025
-
What Is The Lowest Common Multiple Of 10 And 15
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Phosphorus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
