What Is The Electron Configuration For N
listenit
Mar 16, 2025 · 5 min read
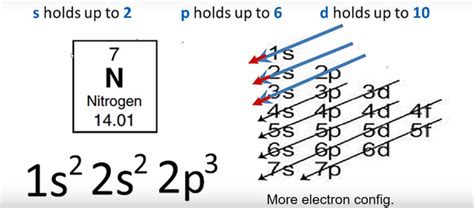
Table of Contents
What is the Electron Configuration for Nitrogen? Understanding Atomic Structure and Orbital Filling
Nitrogen, a crucial element for life as we know it, holds a fascinating position in the periodic table. Its atomic number, 7, dictates its electron configuration, a fundamental property that governs its chemical behavior and reactivity. This article dives deep into understanding nitrogen's electron configuration, exploring the underlying principles of atomic structure and orbital filling, and highlighting its significance in chemistry and beyond.
Understanding Electron Configuration
The electron configuration of an atom describes how electrons are arranged in its various energy levels and sublevels. It's a fundamental concept in chemistry, providing a framework for predicting an element's chemical properties and reactivity. This arrangement follows specific rules based on the quantum mechanical model of the atom, which dictates how electrons occupy atomic orbitals.
Key Principles:
- Aufbau Principle: Electrons fill the lowest energy levels first. This principle ensures that the atom is in its most stable state.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, each with opposite spins (represented as ↑ and ↓).
- Hund's Rule: When filling orbitals of equal energy (degenerate orbitals), electrons will occupy each orbital individually before pairing up. This minimizes electron-electron repulsion.
These principles are crucial for accurately predicting the electron configuration of any element, including nitrogen.
Determining the Electron Configuration of Nitrogen (N)
Nitrogen's atomic number is 7, meaning it has 7 protons and, in a neutral atom, 7 electrons. To determine its electron configuration, we'll follow the Aufbau principle, filling the orbitals in order of increasing energy:
-
1s orbital: The first energy level (n=1) contains only one subshell, the 's' subshell, which can hold a maximum of two electrons. Therefore, nitrogen's two lowest-energy electrons fill this orbital: 1s².
-
2s orbital: The second energy level (n=2) also contains an 's' subshell, which can hold another two electrons. These are the next two electrons added: 2s².
-
2p orbitals: The second energy level also contains three 'p' orbitals (2px, 2py, 2pz), each capable of holding two electrons. This means the 'p' subshell can hold a total of six electrons. Nitrogen has three remaining electrons, which will occupy these 'p' orbitals individually before pairing up (Hund's Rule): 2p³.
Therefore, the complete electron configuration for nitrogen is 1s²2s²2p³.
Visualizing Nitrogen's Electron Configuration
It's often helpful to visualize the electron configuration using orbital diagrams. These diagrams show each orbital as a box, and electrons are represented by arrows. For nitrogen:
- 1s: ↑↓
- 2s: ↑↓
- 2p: ↑ ↑ ↑ (one electron in each 2p orbital before pairing)
This visual representation clearly illustrates the distribution of electrons in nitrogen's orbitals and emphasizes the adherence to Hund's rule.
Significance of Nitrogen's Electron Configuration
Nitrogen's electron configuration is pivotal in understanding its chemical properties and reactivity:
-
Valence Electrons: The outermost electrons, those in the 2s and 2p subshells, are called valence electrons. Nitrogen has five valence electrons (2s²2p³), which are crucial for forming chemical bonds.
-
Chemical Bonding: Nitrogen's five valence electrons enable it to form a variety of covalent bonds. It often forms three covalent bonds to achieve a stable octet (eight electrons in its valence shell), as seen in ammonia (NH₃) and many organic compounds. However, it can also form a triple bond with another nitrogen atom to create the very stable diatomic nitrogen molecule (N₂).
-
Reactivity: The presence of three unpaired electrons in the 2p orbitals makes nitrogen relatively reactive, particularly at high temperatures or with the presence of a catalyst. This reactivity is responsible for its ability to form a wide range of compounds.
-
Biological Significance: Nitrogen's unique electronic structure and chemical behavior underpin its essential role in biological systems. It's a major component of amino acids, the building blocks of proteins, and nucleic acids (DNA and RNA), which carry genetic information. The nitrogen cycle, a crucial biogeochemical cycle, highlights nitrogen's importance in maintaining life on Earth.
Nitrogen's Role in Various Fields
The importance of nitrogen extends beyond its biological role. It is a vital element in numerous industrial processes and applications:
-
Fertilizers: Nitrogen is a key component of fertilizers, supplying essential nutrients for plant growth. The Haber-Bosch process, which synthesizes ammonia from nitrogen and hydrogen, is a cornerstone of modern agriculture.
-
Pharmaceuticals: Nitrogen is a constituent in many pharmaceuticals and medicinal compounds, contributing to their structure and activity.
-
Explosives: Due to its ability to form stable and energetic bonds, nitrogen is used in the manufacture of various explosives.
-
Materials Science: Nitrogen is used in the production of various materials, including advanced ceramics and nitrides, which possess unique properties such as high hardness and corrosion resistance.
Beyond the Basics: Excited States and Ionization
While the 1s²2s²2p³ configuration represents nitrogen's ground state (lowest energy level), it can also exist in excited states. When nitrogen absorbs energy, one or more electrons can jump to higher energy levels. These excited states are less stable and tend to revert to the ground state, often releasing energy in the form of light.
Ionization involves removing electrons from an atom. Nitrogen can lose electrons to form ions, such as N⁺, N²⁺, and so on. The ionization energies increase as more electrons are removed, reflecting the increasing difficulty of removing electrons from increasingly positively charged species.
Conclusion: The Fundamental Importance of Electron Configuration
Understanding the electron configuration of nitrogen, its 1s²2s²2p³, is not merely an academic exercise. It provides a crucial foundation for grasping its chemical behavior, reactivity, and its central role in various aspects of chemistry, biology, and numerous industrial applications. Its five valence electrons, governed by fundamental principles of atomic structure, dictate its capacity for bonding, leading to the formation of a vast array of crucial compounds that are fundamental to life and numerous technological advancements. The implications of its electron configuration are far-reaching, making it a subject of continued study and fascination for scientists and researchers across many disciplines. From the intricate workings of the nitrogen cycle to the synthesis of life-saving pharmaceuticals, nitrogen’s unique electronic structure lies at the heart of it all.
Latest Posts
Latest Posts
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For N . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
