Number Of Valence Electrons In Carbon
listenit
Mar 17, 2025 · 6 min read
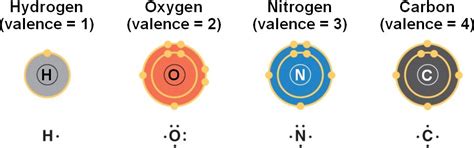
Table of Contents
The Enigmatic Charm of Carbon: Unveiling the Significance of its Four Valence Electrons
Carbon, the cornerstone of organic chemistry and the basis of all known life, holds a unique position in the periodic table. Its remarkable properties, responsible for the incredible diversity of organic molecules, are intrinsically linked to a seemingly simple fact: carbon has four valence electrons. This seemingly small detail underpins an immense complexity, driving the formation of diverse and intricate molecular structures that shape our world. This article will delve deep into the significance of carbon's four valence electrons, exploring their impact on bonding, chemical reactivity, and the emergence of life itself.
Understanding Valence Electrons: The Key to Chemical Bonding
Before diving into the specifics of carbon, let's briefly review the concept of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the electrons most involved in chemical bonding, determining an atom's reactivity and the types of bonds it can form. The number of valence electrons dictates the atom's capacity to share, gain, or lose electrons to achieve a stable electron configuration, typically resembling that of a noble gas. This stable configuration, often characterized by a full outer electron shell, is the driving force behind chemical bonding.
Carbon's Unique Tetravalence: The Foundation of Organic Chemistry
Carbon, with its atomic number 6, possesses an electronic configuration of 1s²2s²2p². This means it has two electrons in the first shell (1s²) and four electrons in the second shell (2s²2p²). The four electrons in the second shell are the valence electrons, making carbon tetravalent. This tetravalency is the cornerstone of carbon's remarkable versatility and the foundation of the entire field of organic chemistry.
The Significance of Four Valence Electrons
The presence of four valence electrons allows carbon to form a maximum of four covalent bonds. A covalent bond is formed when two atoms share one or more pairs of electrons. This ability to form four strong covalent bonds allows carbon to:
-
Form long chains and rings: Carbon atoms can bond with other carbon atoms to form long chains, branched chains, and rings, creating a vast array of complex molecules. This is unlike many other elements, which tend to form shorter chains or simpler structures.
-
Form single, double, and triple bonds: Carbon can form single, double, or triple bonds with other carbon atoms or with other elements, further increasing the diversity of possible molecular structures. These different bond types impact the molecule's geometry, reactivity, and properties.
-
Form stable structures: The four strong covalent bonds formed by carbon contribute to the stability of organic molecules. These molecules are often resistant to degradation and can persist for long periods.
-
Exhibit isomerism: The variety of bonding possibilities leads to isomerism, where molecules with the same chemical formula have different structural arrangements and thus different properties. This further enhances the diversity of organic compounds.
Carbon's Bonding Prowess: A Detailed Look at its Interactions
Let's examine some examples of how carbon's four valence electrons lead to the formation of various molecular structures:
Methane (CH₄): A Simple Yet Crucial Example
Methane (CH₄) is the simplest organic molecule, showcasing carbon's tetravalency perfectly. Carbon shares one electron with each of the four hydrogen atoms, forming four single covalent bonds. The resulting molecule adopts a tetrahedral geometry, with bond angles of approximately 109.5°.
Ethane (C₂H₆): The Beginning of Carbon Chains
Ethane (C₂H₆) demonstrates carbon's ability to form chains. Two carbon atoms share one electron pair, forming a single covalent bond. Each carbon atom then forms three additional bonds with hydrogen atoms. This shows the simple yet fundamental principle of carbon-carbon bonding, allowing for the expansion into larger molecules.
Ethylene (C₂H₄): The Introduction of Double Bonds
Ethylene (C₂H₄) introduces the concept of double bonds. Two carbon atoms share two electron pairs, forming a double bond, alongside two single bonds with hydrogen atoms each. This double bond influences the molecule's geometry and reactivity, leading to different properties compared to single-bonded molecules.
Acetylene (C₂H₂): The Power of Triple Bonds
Acetylene (C₂H₂) illustrates the formation of triple bonds. Two carbon atoms share three electron pairs, creating a triple bond and leaving each carbon atom to form a single bond with a hydrogen atom. This type of bond is even stronger and shorter than double and single bonds, influencing reactivity and properties significantly.
Carbon's Impact on Life: The Building Blocks of Biology
The unique properties stemming from carbon's four valence electrons are not only the basis for organic chemistry; they are also fundamental to the existence of life as we know it. Carbon's ability to form diverse and complex molecules allows for the creation of:
-
Carbohydrates: These essential energy sources and structural components are built from carbon, hydrogen, and oxygen atoms. The long chains and rings formed by carbon atoms provide the structural framework for carbohydrates.
-
Lipids: These fats and oils play crucial roles in energy storage, cell membrane structure, and signaling. The hydrophobic nature of lipids is partly due to the nonpolar carbon-hydrogen bonds.
-
Proteins: Proteins, the workhorses of the cell, are built from amino acids, which contain carbon atoms as their central element. The complex structures and interactions of proteins arise from the diverse bonding possibilities of carbon.
-
Nucleic Acids: DNA and RNA, the carriers of genetic information, are built from nucleotides containing carbon-based sugar and phosphate backbones. The precise sequencing of these carbon-containing units determines the genetic code.
Without carbon's ability to form a vast array of stable and complex molecules, life as we know it would not exist.
Conclusion: The Enduring Significance of Carbon's Four Valence Electrons
The seemingly simple fact that carbon possesses four valence electrons has profound implications across numerous fields. Its ability to form strong covalent bonds, long chains, and complex ring structures, coupled with its capacity to form single, double, and triple bonds, underpins the remarkable diversity of organic molecules and the very existence of life. Understanding the significance of carbon's four valence electrons is crucial for comprehending the fundamental principles of chemistry, biology, and materials science. From the synthesis of new drugs and polymers to the exploration of extraterrestrial life, the enduring legacy of carbon’s tetravalency continues to shape our understanding of the universe and our place within it. The study of carbon and its myriad compounds remains a vibrant and ever-evolving field, promising further breakthroughs and discoveries in the years to come.
Latest Posts
Latest Posts
-
How Much Is 70 Degrees Fahrenheit In Celsius
Mar 18, 2025
-
24 Is What Percent Of 120
Mar 18, 2025
-
A Quadrilateral With Two Pairs Of Parallel Sides
Mar 18, 2025
-
1 4 To The Power Of 2
Mar 18, 2025
-
Why Chemical Equations Must Be Balanced
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
