Number Of Valence Electrons In Aluminium
listenit
Mar 24, 2025 · 6 min read
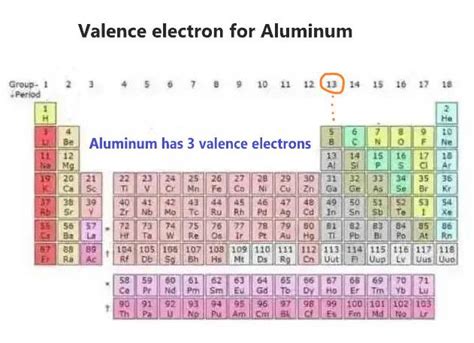
Table of Contents
Unveiling the Secrets of Aluminum: A Deep Dive into its Valence Electrons
Aluminum, a ubiquitous metal found in everything from soda cans to airplanes, holds a fascinating position in the periodic table. Understanding its properties, particularly its valence electrons, is key to comprehending its remarkable versatility and reactivity. This comprehensive guide will delve into the world of aluminum's valence electrons, exploring its electronic configuration, chemical behavior, and the implications for its widespread applications.
What are Valence Electrons?
Before we focus specifically on aluminum, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they determine an atom's chemical behavior and its ability to form bonds with other atoms. They are the primary players in chemical reactions, influencing an element's reactivity, bonding capacity, and overall chemical properties. The number of valence electrons dictates how many bonds an atom can form, whether it will readily gain, lose, or share electrons, and subsequently, its position in the periodic table.
Determining the Number of Valence Electrons in Aluminum
Aluminum (Al) has an atomic number of 13, meaning it possesses 13 protons and, in its neutral state, 13 electrons. To determine the number of valence electrons, we need to examine its electronic configuration. This configuration describes how electrons are distributed among the different energy levels or shells within the atom. Following the Aufbau principle and Hund's rule, the electronic configuration of aluminum is 1s²2s²2p⁶3s²3p¹.
The outermost shell for aluminum is the third shell (n=3), which contains three electrons: two in the 3s subshell and one in the 3p subshell. Therefore, aluminum possesses three valence electrons.
Understanding Electronic Configuration
The electronic configuration is written using a shorthand notation that indicates the principal quantum number (n), the subshell (s, p, d, f), and the number of electrons in each subshell. Let's break down aluminum's configuration:
- 1s²: This signifies two electrons in the first energy level (n=1) within the 's' subshell. The 's' subshell can hold a maximum of two electrons.
- 2s²: This represents two electrons in the second energy level (n=2) within the 's' subshell.
- 2p⁶: This indicates six electrons in the second energy level (n=2) within the 'p' subshell. The 'p' subshell can hold a maximum of six electrons.
- 3s²: This describes two electrons in the third energy level (n=3) within the 's' subshell.
- 3p¹: This represents one electron in the third energy level (n=3) within the 'p' subshell.
The filled inner shells (1s², 2s², 2p⁶) are considered core electrons and do not participate actively in chemical bonding. Only the electrons in the outermost shell (3s²3p¹) are valence electrons and are available for bonding.
Aluminum's Chemical Behavior: The Role of Valence Electrons
The presence of three valence electrons profoundly influences aluminum's chemical behavior. It tends to lose these three valence electrons to achieve a stable octet configuration, similar to that of the noble gas neon. This electron loss results in the formation of a +3 ion (Al³⁺). This tendency to lose electrons makes aluminum a highly reactive metal, especially with oxidizing agents.
Oxidation and Reduction
The process of losing electrons is called oxidation, and aluminum readily undergoes oxidation. Conversely, the process of gaining electrons is called reduction. In chemical reactions, oxidation and reduction always occur simultaneously – a process known as a redox reaction. Aluminum's strong tendency to oxidize makes it an excellent reducing agent, meaning it can readily donate electrons to other substances.
Reactivity with Oxygen and Water
The high reactivity of aluminum is evident in its reaction with oxygen and water. When exposed to air, aluminum rapidly forms a thin, protective layer of aluminum oxide (Al₂O₃). This oxide layer acts as a passivation layer, preventing further oxidation and corrosion. This is a crucial factor in aluminum's widespread use in construction and various applications, despite its inherent reactivity. While the oxide layer protects against further reaction in many environments, strong acids and alkalis can still react with aluminum.
Formation of Ionic Compounds
Aluminum's three valence electrons allow it to form ionic compounds with nonmetals. In these compounds, aluminum loses its three valence electrons to nonmetals, which readily accept these electrons. The resulting ions (Al³⁺ and the negatively charged nonmetal ion) are held together by electrostatic forces of attraction, forming a stable ionic lattice structure. Examples of aluminum's ionic compounds include aluminum chloride (AlCl₃), aluminum oxide (Al₂O₃), and aluminum sulfide (Al₂S₃).
Applications of Aluminum and the Significance of Valence Electrons
The unique properties of aluminum stemming directly from its three valence electrons have led to its widespread use in a vast array of applications. Its lightness, strength, corrosion resistance, and excellent conductivity contribute to its versatility.
Transportation
Aluminum's light weight and strength make it ideal for use in the transportation industry. It is extensively used in the construction of aircraft, automobiles, and trains, contributing to fuel efficiency and reduced weight.
Packaging
Aluminum's malleability and corrosion resistance make it a popular choice for packaging materials. Aluminum foil, cans, and containers are commonly used for food and beverage packaging, providing protection and extending shelf life.
Construction
Aluminum's strength, durability, and corrosion resistance make it a suitable material for construction applications. It is used in building facades, window frames, roofing materials, and various structural components.
Electrical Applications
Aluminum's high electrical conductivity makes it a valuable material in electrical applications. It is used in electrical wiring, transmission lines, and various electrical components.
Other Applications
Aluminum finds use in numerous other applications, including:
- Consumer electronics: Mobile phones, laptops, and other electronic devices utilize aluminum for its lightweight and durable properties.
- Aerospace: Aluminum alloys are used extensively in aircraft and spacecraft construction.
- Medical implants: Aluminum's biocompatibility makes it suitable for certain medical implants.
- Cooking utensils: Aluminum's excellent heat conductivity makes it a popular choice for cookware.
Conclusion: The Importance of Understanding Valence Electrons
The number of valence electrons in aluminum – three – is fundamental to understanding its chemical and physical properties. This seemingly simple number dictates its reactivity, bonding behavior, and overall suitability for a wide array of applications. From the protective oxide layer that prevents widespread corrosion to its ability to form strong alloys, the three valence electrons of aluminum are central to its unique characteristics and its indispensable role in modern technology and everyday life. Further research into aluminum's properties continues to expand its applications and highlight the crucial role its valence electrons play in its remarkable utility. A complete understanding of this fundamental aspect of its atomic structure enables us to harness its capabilities effectively and efficiently. The information provided here serves as a springboard for further exploration into the fascinating world of materials science and chemistry.
Latest Posts
Latest Posts
-
How Many Light Years Is Pluto From Earth
Mar 29, 2025
-
The Combination Of All Forces Acting On An Object
Mar 29, 2025
-
What Does A Subscript In A Chemical Formula Represent
Mar 29, 2025
-
23 Out Of 30 Is What Percent
Mar 29, 2025
-
What Type Of Speech Is Is
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Aluminium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
