What Does A Subscript In A Chemical Formula Represent
listenit
Mar 29, 2025 · 6 min read
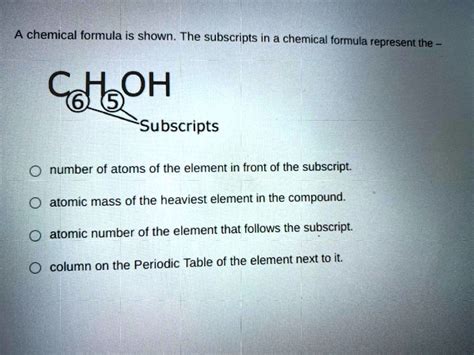
Table of Contents
What Does a Subscript in a Chemical Formula Represent? A Deep Dive into Chemical Notation
Understanding chemical formulas is fundamental to grasping the principles of chemistry. These formulas, at their core, represent the composition of a substance, telling us which elements are present and in what proportions. A critical component of chemical formulas is the subscript, a small number written slightly below and to the right of an element's symbol. But what exactly does this subscript represent? This article delves deep into the meaning and significance of subscripts in chemical formulas, exploring their implications for stoichiometry, balancing equations, and understanding chemical reactions.
The Fundamental Role of Subscripts: Indicating Atom Ratios
At its most basic level, a subscript in a chemical formula indicates the number of atoms of a specific element present in one molecule or formula unit of a compound. For instance, consider the formula for water: H₂O. The subscript '2' after the hydrogen symbol (H) signifies that each molecule of water contains two atoms of hydrogen. The absence of a subscript after the oxygen symbol (O) implicitly means there is one atom of oxygen in each water molecule.
This seemingly simple concept has profound implications. It forms the bedrock of stoichiometry, allowing us to:
- Determine the relative abundance of elements: From the formula, we can immediately deduce that water is composed of two parts hydrogen to one part oxygen by atom count.
- Calculate molar masses: Knowing the number of atoms of each element in a molecule, we can calculate its molar mass (the mass of one mole of the substance) using the atomic masses of the constituent elements.
- Understand chemical reactions: Subscripts are crucial for balancing chemical equations, ensuring that the number of atoms of each element remains consistent on both sides of the equation.
Beyond Simple Molecules: Subscripts in More Complex Formulas
The significance of subscripts expands beyond simple diatomic or triatomic molecules. Consider the formula for glucose, C₆H₁₂O₆. Here, the subscripts indicate:
- 6 carbon atoms
- 12 hydrogen atoms
- 6 oxygen atoms
This formula reveals the precise atomic composition of a glucose molecule, vital information for understanding its properties, reactions, and metabolic pathways. Similarly, complex ionic compounds also utilize subscripts to represent the ratio of ions in the crystal lattice. For example, the formula for magnesium chloride, MgCl₂, shows that for every magnesium ion (Mg²⁺), there are two chloride ions (Cl⁻). The subscripts reflect the charge balance necessary for the formation of a neutral compound.
Polyatomic Ions and the Use of Parentheses
When dealing with polyatomic ions (ions composed of multiple atoms), parentheses are used to enclose the ion, followed by a subscript indicating the number of those ion units present. For example, the formula for calcium phosphate, Ca₃(PO₄)₂, showcases this:
- 3 calcium ions (Ca²⁺)
- 2 phosphate ions (PO₄³⁻)
The parentheses ensure that the subscript '2' applies to the entire phosphate ion (PO₄), indicating two complete phosphate units, each containing one phosphorus atom and four oxygen atoms. Without the parentheses, the formula would be misinterpreted.
Subscripts and Empirical vs. Molecular Formulas
It's crucial to differentiate between empirical and molecular formulas. An empirical formula represents the simplest whole-number ratio of atoms in a compound. A molecular formula, on the other hand, represents the actual number of atoms of each element present in a molecule.
For example, the empirical formula for glucose is CH₂O, reflecting the 1:2:1 ratio of carbon, hydrogen, and oxygen atoms. However, the molecular formula, C₆H₁₂O₆, reveals the actual number of atoms in one glucose molecule, which is six times the empirical formula. The subscripts in the molecular formula provide the complete picture of the molecule's composition.
Determining the molecular formula often requires additional information, such as the molar mass of the compound. Once the molar mass is known, it can be compared to the molar mass calculated from the empirical formula to determine the integer multiple by which the empirical formula must be multiplied to obtain the molecular formula.
The Significance of Subscripts in Chemical Reactions and Equations
Subscripts play a pivotal role in balancing chemical equations, a fundamental aspect of stoichiometry. A balanced chemical equation ensures that the number of atoms of each element is conserved throughout the reaction. This is achieved by adjusting the coefficients (numbers placed in front of chemical formulas) while keeping the subscripts within each formula constant.
Consider the reaction between hydrogen and oxygen to form water:
2H₂ + O₂ → 2H₂O
Here, the subscripts within the formulas (H₂, O₂, and H₂O) remain unchanged. The coefficients (2, 1, and 2) are adjusted to balance the equation, ensuring that there are four hydrogen atoms and two oxygen atoms on both sides of the arrow. Changing subscripts would alter the chemical identity of the compounds involved, leading to an unbalanced and incorrect representation of the reaction.
Interpreting Subscripts: A Practical Approach
To accurately interpret subscripts in chemical formulas, follow these steps:
- Identify the elements: Recognize the chemical symbols representing each element in the formula.
- Examine the subscripts: Note the subscript following each element symbol. If no subscript is present, it is understood to be 1.
- Determine the number of atoms: The subscript indicates the number of atoms of that element in one molecule or formula unit of the compound.
- Consider polyatomic ions: If parentheses are used, the subscript outside the parentheses applies to the entire ion within.
- Distinguish empirical and molecular formulas: Understand the difference between representing the simplest ratio (empirical) and the actual number of atoms (molecular).
Subscripts and Beyond: Other Chemical Notations
While subscripts are crucial for conveying the composition of substances, they are only one part of a broader system of chemical notation. Other notations, such as:
- Coefficients: Indicate the number of molecules or formula units involved in a reaction.
- Superscripts: Often used to represent charges on ions (e.g., Na⁺, Cl⁻).
- Brackets: Used in coordination chemistry to denote ligands surrounding a central metal ion.
All these notations work together to create a comprehensive and unambiguous system for representing chemical compounds, reactions, and processes.
Conclusion: The Unsung Hero of Chemical Notation
The seemingly small subscript plays a monumental role in the world of chemistry. Its ability to precisely define the atomic composition of substances is fundamental to understanding chemical reactions, calculating molar masses, balancing equations, and mastering stoichiometry. From simple molecules to complex ionic compounds, the subscript remains a constant and indispensable component of chemical formulas, serving as a cornerstone of chemical communication and analysis. A thorough understanding of subscripts is essential for anyone aspiring to comprehend and contribute to the field of chemistry. Mastering this basic concept unlocks a deeper appreciation for the intricacies of the chemical world and its elegant system of notation.
Latest Posts
Latest Posts
-
What Is The Highest Point Of A Transverse Wave Called
Mar 31, 2025
-
Diameter Of The Solar System In Light Years
Mar 31, 2025
-
48 Of 60 Is What Percent
Mar 31, 2025
-
Is Adenine A Purine Or Pyrimidine
Mar 31, 2025
-
A Quadrilateral With Opposite Sides Parallel
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Does A Subscript In A Chemical Formula Represent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
