Number Of Valence Electrons In Al
listenit
Mar 16, 2025 · 5 min read
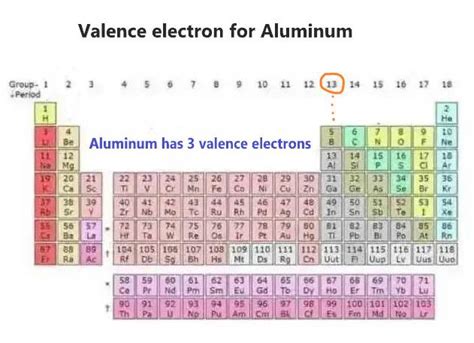
Table of Contents
Unveiling the Valence Electrons of Aluminum: A Deep Dive into Atomic Structure and Chemical Behavior
Aluminum, a ubiquitous metal found in everything from beverage cans to aircraft components, possesses a fascinating atomic structure that dictates its unique chemical properties. Understanding the number of valence electrons in aluminum is key to grasping its reactivity, bonding capabilities, and overall role in the world around us. This comprehensive guide delves into the intricacies of aluminum's electron configuration, explaining why it exhibits the behavior it does and highlighting its importance in various applications.
What are Valence Electrons?
Before we explore aluminum specifically, let's define valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the electrons most involved in chemical reactions, determining an element's reactivity and bonding preferences. Valence electrons are crucial because they dictate how an atom interacts with other atoms, forming molecules and compounds. The number of valence electrons directly influences an element's oxidation state and its ability to gain, lose, or share electrons to achieve a stable electron configuration.
Determining the Number of Valence Electrons in Aluminum (Al)
Aluminum, with the atomic symbol Al and atomic number 13, possesses 13 protons and 13 electrons in its neutral state. To determine its number of valence electrons, we need to examine its electron configuration. This configuration describes how electrons are distributed among the different energy levels or shells within the atom.
Electron Configuration of Aluminum
The electron configuration of aluminum is 1s²2s²2p⁶3s²3p¹. This notation tells us the following:
- 1s²: Two electrons occupy the first energy level (shell), in the 's' subshell.
- 2s²: Two electrons occupy the second energy level, in the 's' subshell.
- 2p⁶: Six electrons occupy the second energy level, in the 'p' subshell.
- 3s²: Two electrons occupy the third energy level, in the 's' subshell.
- 3p¹: One electron occupies the third energy level, in the 'p' subshell.
The outermost shell, or valence shell, for aluminum is the third energy level (n=3). This shell contains a total of three electrons (two in the 3s subshell and one in the 3p subshell). Therefore, aluminum has three valence electrons.
The Significance of Three Valence Electrons
The presence of three valence electrons is pivotal in understanding aluminum's chemical behavior. Aluminum readily loses these three electrons to achieve a stable octet configuration, mimicking the electron arrangement of the noble gas neon (Ne). This tendency to lose electrons makes aluminum a highly reactive metal, readily participating in chemical reactions and forming stable ionic compounds.
Aluminum's Chemical Behavior: A Consequence of its Valence Electrons
The three valence electrons of aluminum have profound implications for its chemical and physical properties.
Reactivity and Oxidation
Aluminum's high reactivity stems from its strong tendency to lose its three valence electrons. This electron loss results in the formation of a +3 aluminum ion (Al³⁺), which is a stable cation. This oxidation process is evident in the reaction of aluminum with oxygen in the air, forming a protective layer of aluminum oxide (Al₂O₃). This oxide layer passivates the aluminum, preventing further oxidation and contributing to its corrosion resistance.
Bonding Characteristics
Aluminum primarily forms ionic bonds, losing its three valence electrons to electropositive elements like chlorine, sulfur, or oxygen. These ionic bonds result in the formation of stable compounds, such as aluminum chloride (AlCl₃), aluminum sulfide (Al₂S₃), and aluminum oxide (Al₂O₃). Aluminum can also participate in covalent bonding, but this is less common than ionic bonding. In covalent bonding, it shares its valence electrons with other atoms.
Formation of Alloys
Aluminum's ability to readily form alloys with other metals is a direct consequence of its three valence electrons. These alloys often exhibit enhanced properties compared to pure aluminum, such as increased strength, hardness, and corrosion resistance. Common aluminum alloys include those with copper, magnesium, silicon, and zinc. These alloys find extensive use in various applications, such as transportation (aircraft, automobiles), packaging, construction, and electronics.
Applications Leveraging Aluminum's Valence Electrons
The unique properties of aluminum, stemming from its three valence electrons, have led to its widespread use across numerous industries:
- Packaging: Aluminum's lightweight nature, corrosion resistance, and malleability make it ideal for creating cans, foils, and other packaging materials.
- Transportation: Aluminum alloys are extensively used in the aerospace and automotive industries because of their high strength-to-weight ratio and corrosion resistance.
- Construction: Aluminum is used in building materials such as window frames, doors, and cladding due to its lightweight nature, durability, and aesthetic appeal.
- Electrical Conductivity: Aluminum's high electrical conductivity makes it a suitable material for electrical wiring and transmission lines.
- Heat Transfer: Aluminum's excellent heat conductivity makes it ideal for use in heat sinks and cookware.
Aluminum's Role in Everyday Life
Aluminum's prevalence in our daily lives is undeniable. From the cans holding our beverages to the smartphones we use, aluminum's properties, largely dictated by its three valence electrons, are integral to modern technology and infrastructure. Its lightweight nature, corrosion resistance, and formability continue to drive innovation across various sectors, ensuring its enduring importance.
Conclusion: The Importance of Understanding Valence Electrons
Understanding the number of valence electrons in an element is fundamental to predicting its chemical behavior and applications. Aluminum, with its three valence electrons, serves as a prime example of how this simple number dictates the element's reactivity, bonding preferences, and ultimately, its widespread use in diverse technological applications. By understanding the connection between atomic structure and macroscopic properties, we gain a deeper appreciation for the materials that shape our world. The seemingly simple concept of valence electrons holds the key to understanding the complex world of chemistry and material science, unlocking the potential for innovation and advancement. The study of aluminum's valence electrons serves as an excellent entry point into this fascinating field, demonstrating the power of fundamental scientific principles.
Latest Posts
Latest Posts
-
33 Is 22 Of What Number
Mar 16, 2025
-
Find The Area Of The Parallelogram With Vertices
Mar 16, 2025
-
Number Of Electrons In Carbon 12
Mar 16, 2025
-
Slope Intercept Form With Undefined Slope
Mar 16, 2025
-
How To Solve Equations With Feet
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Al . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
